About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Materials of the conference "EDUCATION AND SCIENCE WITHOUT BORDERS"
Currently, one of such problem is the pollution of soil by various kinds of pollutants, especially dangerous - soil pollution by stable, toxic components such as heavy metals (HM). A large proportion of gas and dust emission is deposited on the surface of the soil, at that, the most fertile top layer is polluted. In reaction of soils to a technogenic stress, in their evolution from natural state to technologically impaired, there is an accumulation of chemical pollutants to a critical level, as well as significant changes in the physical and chemical properties of soils. As metal of study was selected the lead because it is a priority pollutant of East Kazakhstan, including the Semipalatinsk PriIrtysh. The problem of lead has an important ecological aspect. It belongs to the HM of I danger class, characterized by high toxicity, mutagenic and cancerogenic effect, is capable to bio-accumulation. High concentrations of lead in the environment are harmful to the ecosystem, while the lowest are vital to living organisms as a microcomponent. Lead pollution of soil has long-lasting (the half-removing of lead ranges from 740 to 5900 years), so the study of the absorption properties of the soils at different levels of technogenic impact - this is a serious ecological problem, an important in scientific and practical aspects.
Its decision will assess the ecological condition of the soil cover, and develop a variety of methods to reduce or eliminate the consequences of pollution.
In the study area are such large metallurgical enterprises as Zhezkent Mining, Ziryanovsk lead plant, Ust-Kamenogorsk lead-zinc plant, Leninogorskiy polymetallic plant, Ulbinsk metallurgical plant, and the Semipalatinsk ship-repair and shipbuilding plant, from which the surface of the Earth each year received 89 tons of lead. The burning of coal and oil in power plants of the region on the surface of the Earth receives 3600 tons of lead per year. Other, not less dangerous sources of soil pollution of lead compounds, mining, ore processing, metalworking, machine building and chemical industry, and motor vehicles, the exhaust gases which bring to the surface of the Earth by different estimations from 180 thousand tons to 260 thousand tons of lead particles. With constant introduction of high doses of mineral and organic fertilizers that contain HM, in the soil also can enter their significant amounts1.
In agriculture, for many years been widely used technology intensive cultivation, including high doses of fertilizer, as the use of organic and mineral fertilizers - one of the basic conditions for improving crop yields, as well as an important link technology to grow them. Fertilization largely changes chemistry element in soil by flowing in the ground an additional amount of metal and by changing the mobility of the element in the soil. Chemicals used in agriculture, can change the reaction of the medium, being physiologically acidic or physiologically alkaline, and that, consequently, leads to a change in solubility of the HM compounds. The components of agro-chemicals can change the mobility of metals by absorption, ion exchange and complex formation. Concretion of hydroxides of metals with components of fertilizers not excluded. Buffering capacity of the soil in relation to the HM is mainly connected with the processes occurring at the interface. The main mechanism of absorption of HM by soil – specific adsorption with outward coordination compounds formation. Donor-acceptor interactions are characteristic of nutrient and ameliorants. These processes are accompanied by the release of different structural groups of absorbent surfaces that can not affect the properties of surfaces and, consequently, on specificity, occurring in them, chemical reactions involving compounds of the solution. The absorptive capacity of the soil in relation to the HM depends on the soil properties such as particle size distribution and mineralogical composition, the content of organic matter, carbonate, pH, cation exchange capacity, etc., as well as the chemical properties of the HM. HM ions that are in the soil solution can be absorbed on the surface of the different components of the soil, and it will be the defining element of their future behavior in the soil. The mobility of heavy metals in soils is influenced by processes such as adsorption-desorption, precipitation-dissolution, ion exchange, diffusion, etc. All above-mentioned processes create a complex picture of the behavior of HM ions in the soil, and therefore, there are difficulties in the calculations.
Considering that completely there are no experimental data about lead absorption by soils of Kazakhstan, our studies have focused on this aspect, which will provide an ecological assessment of their resistance to lead pollution. The objects of the study were used humus horizons (0-20 cm) of chestnut soils that are common in the Semipalatinsk PriIrtysh: typical leached slightly argillaceous loam, typical leached slightly argillaceous medium loamy and solonetzic leached slightly argillaceous medium loamy (soil types listed according to the classification1). Samples were taken in areas not subject to technogenic impact. This suggests that the data obtained as a result of research on absorbency of the soil is not understated, because the soil was not initially polluted by lead. Sampling and determination of physical and chemical properties of the studied soils was carried out by standard methods1,2,3. Physical and chemical characteristics of the studied soils are as follows (Table 1):
Table 1. Physical and chemical characteristics of soils
|
Soils |
рН |
Humus, % |
Silt, % |
Physical clay, % |
CEC,mg-ekv/100g |
|
1 |
7,2 |
0,9 |
10,1 |
15,2 |
9,5 |
|
2 |
7,0 |
2,5 |
15,3 |
26,1 |
17,8 |
|
3 |
6,9 |
2,3 |
19,5 |
28,9 |
20,4 |
Note: 1 - typical leached slightly argillaceous loam soil; 2 - typical leached slightly argillaceous medium loamy soil; 3 - solonetzic leached slightly argillaceous medium loamy soil.
In general, soils are neutral pH of about 7; poor in humus (the poorest are typically leached slightly argillaceous loam soils), are composed of various amounts of silt and physical clay (the highest concentration of these components is observed in solonetzic leached slightly argillaceous medium loamy soils). Revealed significant differences in the cation exchange capacity. Gross contents and lead compounds in soils are shown in Table 2.
Table 2. Ion concentrations Pb2 +, mg / kg
|
Soils |
Gross content |
Forms of the compounds |
||
|
water-soluble |
acid-soluble |
ion-exchange |
||
|
1 |
13,8-14,5 14±0,15 |
0,047-0,055 0,05±0,012 |
0,76-1,2 1±0,08 |
0,45-0,56 0,5±0,01 |
|
2 |
15,7-16,2 16±0,1 |
0,11-0,15 0,1±0,015 |
1-1,6 1,2±0,075 |
0,33-0,47 0,4±0,025 |
|
3 |
16,5-17,7 17±0,09 |
0,1-0,17 0,1±0,015 |
1,2-1,5 1,3±0,07 |
0,27-0,45 0,4±0,15 |
Note (here and further): in numerator – limits of fluctuations of min – max (mg/kg); in denominator - an arithmetic average – M and its error – m (mg/kg).
The gross content of lead fluctuates ranging from 14 to 17 mg/kg. The most lead-rich solonetzic leached slightly argillaceous medium loamy soils, the least - typical leached slightly argillaceous loam soils.
Mobile Fund of lead compounds is in the range of 10,5 to 11,5% of the gross content, and water-soluble forms have 0,5 - 0,6%, exchangeable forms – 2,5 - 3%, acid-soluble forms – 7,5 - 8%.
Data for the study of the dynamics of absorption of lead by soils are shown in Figure 1.
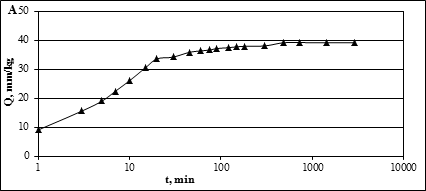
Fig. 1. Dynamics of absorption of lead by soils
(▲ – typical leached slightly argillaceous medium loamy soil)
As seen from the above presented figure, up to a point with increase in reaction time of solid and liquid phase increases the amount of absorbed lead. After that, there is a gradual alignment of graphics, the amount of metal absorbed by the soil eventually stops changing. There comes balance in the system "soil-solution" is that in the context of the experiment is 5-8 hours. The rate of absorption of lead ground during the period preceding the balance of the system is also not constant over time gradually decreases. On the curves it is possible to mark three stages with markedly different angle of slope. The first site corresponds to the first 15 minutes of interaction of the soil with solution.
The second section describes the interaction period of from 15 minutes to 1 hour, and the third - from 1 hour to 5 hours. Specific rate of lead absorption by soil in these intervals were calculated by size of angle of slope tangents marked to the curve linear sections. (Table 3).
Table 3. The rate of lead absorption by soil
|
Section on a curve |
Rate of absorption, mM/g soils per minute |
|
typical leached slightly argillaceous medium loamy soil |
|
|
I |
1,467 |
|
I I |
0,073 |
|
I I I |
0,0055 |
The initial stage of the interaction of soil with the solution is characterized by the highest rate of lead absorption. Comparison of the constants indicates that the rate of lead absorption in the first 15 minutes of its interaction with the soil is 20 times higher than in the next 45 minutes, and 266 times higher than the pre-equilibrium period.
Regularities of changes the rate of lead absorption from solution are explained by the basic laws of chemical kinetics. In the initial moments of the interaction of soil with the solution on the surface of the solid phase there is a large number of various vacant absorption centers of different nature. With increase of time of phase contact number of these position gradually decreases as the binding them by lead ions. Simultaneously in solution reduces steady-state density of lead ions due to their absorption by soil. Thus, reducing the rate of metal absorption by soil from the solution over time, primarily occurs because of filling of absorption centers and by reducing the concentration of the reactants. Some authors have noted the possible role of changing the nature of the absorption centers of surface of the solid phase as well as the role of diffusion in determining the rate of metal absorption by soils1.
In the study of the dynamics of lead absorption by soils was carried out in parallel determination of size changes of pH.
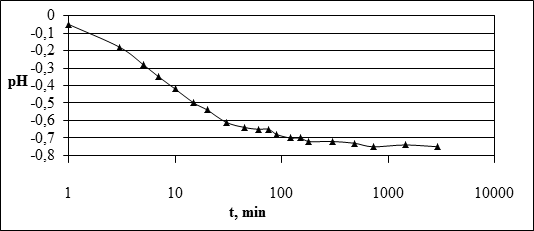
Fig. 2. Dynamics of changes in pH in the process of lead absorption by soil
As shown in Figure 2, the absorption of lead in soil depending on the time pH of solutions decreases. It allows to make the conclusion that at pollution of soils by compounds of lead the ecological situation in a soil profile worsens not only because of presence of toxic ions of metal, but also because of acidification of soil solution. The most dramatic decrease in pH is observed in the first 15 minutes of interaction of soil with the solution, the period of 15 minutes before 1 hour is characterized by a more gradual change in Ph.
Selection of dominant mechanism of lead absorption on the surface of the solid phase can be carried out proceeding from the following regularities. Absorption of lead by method of cation exchange mechanism, i.e. in surface regions with a constant negative charge due to isomorphous substitutions of cations of crystal lattice, by cations of a lower valency, described by a linear absorption isotherm characterized by: high positive degree of absorption capacity depending on the solid phase metal concentrations in the aqueous solution, and the weak dependence on the pH conditions of the environment. Lead absorption on the mechanism of superficial complex formation, i.e. binding of ions on the centers of absorption of the surface, arising owing to processes of protonation and dissociation of protons, is characterized by high positive degree of dependence from рН and weak dependence on the ionic force of solution9. Mainly linear character of isotherms of absorption of lead the soil in experiments with constant value рН and at various concentrations of solutions, as well as increasing the absorption capacity of the soil with increasing pH of the medium (Fig. 3) suggest that the lead absorption in studied soil is the result of combined action, both cation exchange mechanism, and the mechanism of superficial complex formation.
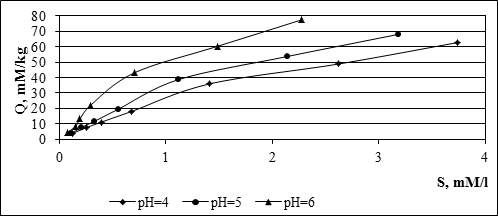
Fig. 3. Isotherms of lead absorption by soil
The results of investigation of the processes of mono and polyelemental absorption of HM of chestnut typical leached slightly argillaceous medium loam soils are presented in the form of isotherms of absorption in Fig. 4.
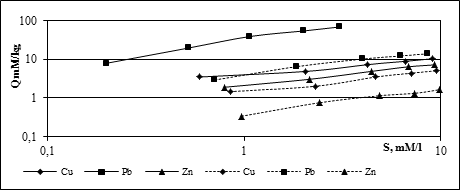
Fig. 4. Isotherms of HM absorption by sol during mono- and polyelemental pollution
(── - monoelemental pollution; --- - polyelemental pollution)
As you can see in Figure 4, during monoelemental pollution there is detected marked differences in the absorption of HM by soil. According to the number of absorbed substances, studied elements can be arranged in the following decreasing series: Pb> Cu> Zn. During absorption the soil accumulates Pb 6.5 times more than Cu, and 9.5 times more than Zn. Obtained results allow to conclude that from the ecological point of view, more dangerous is the pollution of soils by zinc compounds that show the greatest mobility in comparison with the compounds of copper and lead. But it should not be forgotten that the lead refers to the HM of I danger class, therefore presents a significant ecological threat to the environment, even at concentrations lower than copper and zinc.
In polyelemental pollution absorption capacity of the soil is significantly reduced. Pb absorbed 5 times less, Cu - 2 times, Zn - 4 times. The element order by the number of absorbed substances remains the same: Pb> Cu> Zn.
Thus, the polyelemental pollution of soils of HM represents great ecological threat than monoelemental.
2. Kabata-Pendias, A., Pendias H. Trace elements in soils and plants. –M: Mir, 1989. - 439 p.
3. Karavanova E.I, Schmidt S.Y Sorption of water-soluble compounds of copper and zinc by forest litter / / Soil Science. - 2001. - № 9. - P. 1083-1091.
4. Russian soil classification / Compiled by L.L Shishov, V.D Tonkonogov, I.I Lebedeva. - M.: Soil Institute named after V.V Dokuchaev RAAS, 1997. - 236 p.
5. Malinovskii D.N. The adsorption of Sr (II), Cd (II) and Pb (II) on the quaternary deposits of Khibinsk Massif / / Geochemistry. - 2002. - № 4. - S. 426-432.
6. Panin M.S. The forms of heavy metal compounds in soils of central eastern Kazakhstan (background). - Semipalatinsk: "Semey" SU. - 1999. - 329 p.
7. Pinskii D.L. Ion-exchange processes in soils. - Pushchino: DSTI PSC RAS, 1997. - 166.
8. Sadovnikov L.K. Problems of use and recultivation of soils polluted by heavy metals / / Chemicals in Agriculture. - 1995. - № 1. - S. 37-38.
Abduazhitova Assel Muratovna SOIL RESISTANCE TO POLLUTION BY STABLE TOXIC COMPONENTS. International Journal Of Applied And Fundamental Research. – 2013. – № 2 –
URL: www.science-sd.com/455-24422 (20.04.2024).











 PDF
PDF