About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
Abstract
The paper describes a study of different intergel systems sorption capacity in depedence of initial hydrogels molar ratios. Electrochemical properties of solutions which contain ions of gold and lanthanum were studied by measurement of specific eletric conductivity and pH. Volume – gravimetric properties of hydrogels were studied by gravimetric method. In the result of hydrogels remote interaction there is significant increase in sorption capacity relating to metal ions. Due to hydrogels mutual activation there is conformational changes in hydrogels structure. Similar studies allow predicting intergel systems selectivity areas by determining hydrogels molar ratios, which promote their mutual activation. Maximum sorption of gold ions occurs at gPAA:gP2M5VP = 4:2 at 24 hours of interaction.
Keywords: La3+ ions, Au3+ ions, selective sorption, intergel system
Introduction
No wonder all over the world in recent years the demand for rare and rare earth metals increased sharply. It occurs due to it’s intensified role in leading branches of industry, ensuring economic and defensive safety of any state.
Republic of Kazakhstan – is one of the largest regions in the world, which has significant reserves and prospects for expanding of the mineral resource base of rare and rare earth metals. Today, however, the production of rare metlas, rare earth metals and their compounds in Kazakhstan can be characterized as unstable, not corresponding to its potential. In some enterprises production of these metals decreased and suspended.
Meanwhile, in view modern and perspective requirements of science and technology development in the world demand for rare metal and rare earth products increases, and production of clean rare and rare earth metals and their compounds is highly profitable. Hence, for Republic of Kazakhstan priority direction in future – production, selection, production of pure rare and rare earth metals and their compounds.
Previous studies showed that in result of remote interaction hydrogels, which are in intergel systems, have significant changes in volume-gravimetric, sorption properties [1-3]. Hydrogels long-range effect (Jumadilov effect) may be a basis for creation of new technologies in hydrometallurgy, wastewater treatment, industrial and biological solutions, and in other areas, in which there is a necessity in selective separation, concentration and extraction of different charged particles.
Materials and Methods
Equipments
For measurement of electroconductivity conductometer "MARK 603" (Russia) was used, рН of solutions was measured on рН meter "Seven Easy" (METTLER TOLEDO, China). Swelling coefficient Кsw was defined by weighting of hydrogel swollen samples on electronic scales "SHIMADZU AY220" (Japan), Concentration of Au3+ ions was determined by atomic absorption spectroscopy (Varian AA240FS Fast Sequential Atomic Absorption Spectrometer, Australia).
Materials
Studies were carried out in 0.005 M La(NO3)3·6H2O and 0.005 M HAuCl4 solutions. Polyacrylic acid hydrogels were synthesized in the presence of crosslinking agent N,N-methylene-bis-acrylamide and redox system K2S2O8, Na2S2O3. Poly-2-methyl-5-vinylpyridine and poly-4-vinylpyridine hydrogels was synthesized by crosslinking of a linear poly-2-methyl-5-vinylpyridine and poly-4-vinylpyridine in dimethylformamide (DMFA) medium in presence of epichlorohydrin (ECH).
Methods
Experiments were carried out at a room temperature. Initial state of hydrogels was dry. Study of intergel systems was made by this way: each hydrogel was located in separated glass weighing bottle pores of which are permeable for low-molecular ions and molecules, but it is not permeable for a dispersion hydrogels.
Then weighing bottles with hydrogels were located in glasses with distilled water. Electroconductivity and pН of overgel liquid was measured by taking out of weighing bottles with hydrogels from the glass. Swelling coefficient was calculated as the difference of weights of weighing bottle with hydrogel and empty weighing bottle according to Eq. (1).
![]() (1)
(1)
where m1—weight of dry hydrogel, m2—weight of swollen hydrogel
Results and discussion
Sorption of La3+ ions by intergel system gPAA:gP4VP
Obtained dependencies of gPAA:gP4VP intergel system various molar ratios from time are shown on fig. 1, from which it can be seen that specific electric conductivity increases for all ratios with time. However, character of electric conductivity change for various molar ratios if different. With increase of intergel system interaction with lanthanum nitrate solution areas of maximum and minimum electric conductivity appears. After 3 hours of remote interaction minimums at ratios 1:5 and 0:6 become noticeable. At hydrogels ratio 4:2 there is an increase in specific electric conductivity.
In result of previous studies (Jumadilov et. al., 2014a, 2014b) it was established, that electrochemical and conformational properties of hydrogels during remote interaction are in direct dependence of it’s nature.
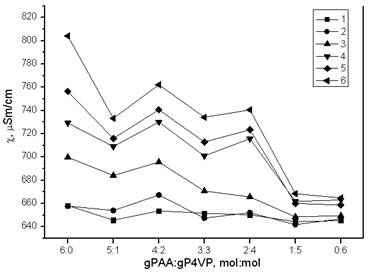
Curves’ decription (interaction time): 1 – 0.0833 h; 2 – 1 h; 3 – 3 h; 4 – 6 h; 5 – 24 h; 6 – 48 h
Figure 1. Dependence of specific electric conductivity from gPAA:gP4VP molar ratios in time
At interaction time 1, 24 and 48 hours dependencies specific electric conductivity changes significantly. At 1 hour maximum of it was at ratio 4:2, at 24 hours it shifted to area 6:0, and at 48 hours it become more distinct.
Minimum values of specific electric conductivity are due to a binding of cleaved proton from carboxyl group by nitrogen heteroatom. High conductivity values indicate that at certain ratios there is domination of carboxyl groups dissociation process over proton association process by nitrogen heteroatoms.
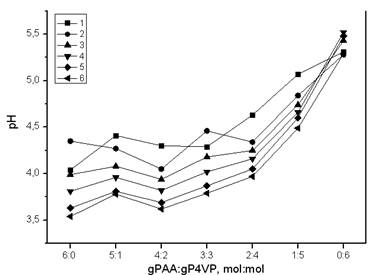
Curves’ decription (interaction time): 1 – 0.0833 h; 2 – 1 h; 3 – 3 h; 4 – 6 h; 5 – 24 h; 6 – 48 h
Figure 2. Dependence of pH from gPAA:gP4VP molar ratios in time
Figure 2 shows changes in hydrogen ions concentration in lanthanum nitrate solution in presence of intergel system gPAA:gP4VP. As it can be seen, at initial moment of time pH of solution has low values, when there is predominance of polyacid. With polyvinylpyridine share increase pH of solution gradually increases, and reaches maximum value at ratio gPAA:gP4VP=0:6. From all curves it is seen, that there is decrease in hydrogen ions concentration with time.
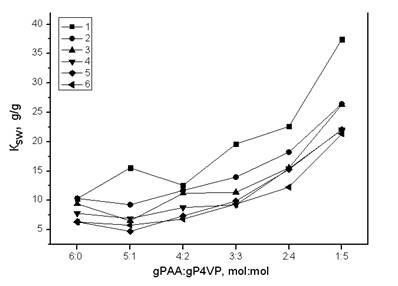
Curves’ decription (interaction time): 1 – 0.0833 h; 2 – 1 h; 3 – 3 h; 4 – 6 h; 5 – 24 h; 6 – 48 h
Figure 3. Dependence of polyacrylic acid swelling coefficient from gPAA:gP4VP molar ratios in time
Polyacid swelling coefficient change is a result of polymer hydrogels long-range effect (fig. 3). There is a significant increase of polyacid swelling when there is increase of polyvinylpyridine share. Area of maximum swelling is hydrogels ratio 1:5. With increase of interaction time there is an decrease of polyacrylic acid swelling coefficient. This results evidence to a phenomena of La3+ ions sorption. When there is a decrease of polyacid share there is a decrease of lanthanum ions sorption, and as a result, there is swelling coefficient increase.
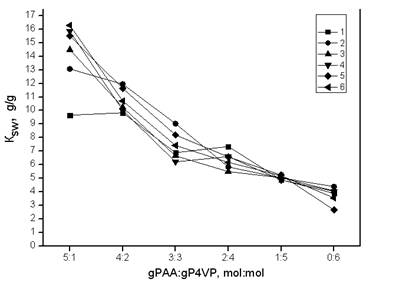
Curves’ decription (interaction time): 1 – 0.0833 h; 2 – 1 h; 3 – 3 h; 4 – 6 h; 5 – 24 h; 6 – 48 h
Figure 4. Dependence of poly-4-vinylpyridines swelling coefficient from gPAA:gP4VP molar ratios in time
Poly-4-vinylpyridines swelling coefficient change in presence of polyacrylic acid is reflected on fig. 4. General type of curves shows increase of polybasis swelling in presence of polyacid. Maximum area of cationic hydrogel swelling at all values of interaction time appears at hydrogels ratio 5:1. With increase of polyvinylpyridine share swelling decreases and reaches minimum at ratios 0:6.
Sorption of Au3+ ions by intergel system gPAA:gP2M5VP
Dependence of gold ions sorption by intergel system gPAA:gP2M5VP from initial hydrogels molar ratios in time is shown on figure 5. As it is seen maximum sorption occurs at ratio gPAA:gP2M5VP = 4:2 at 24 hours of hydrogels remote interaction.
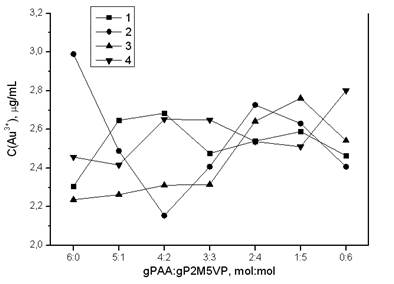
Curves’ decription (interaction time): 1 – 6 h; 2 – 24 h; 3 – 48 h; 4 – 96 h
Figure 5. Dependence of Au3+ ions at sorption of it by intergel system polyacrylic acid hydrogel – poly-2-methyl-5-vinylpyridine hydrogel from HAuCl4 water solutions from gPAA:gP2M5VP molar ratios in time
In this regard a necessity to study sorption kinetics at this hydrogel ratio appeared (fig. 6). It can be seen that maximum amount of gold is recovered by intergel system at 24 hours of interaction.
After this time conformational changes of hydrogels provide slight desorption of gold.
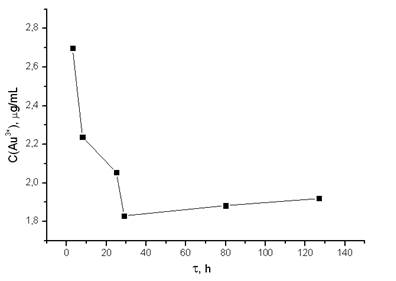
Figure 6. Dependence of Au3+ ions at sorption of it by intergel system polyacrylic acid hydrogel – poly-2-methyl-5-vinylpyridine hydrogel (molar ratio 4:2) from HAuCl4 water solutions from time
Conclusion
Application of intergel systems in industry provides several advantages over existing analogues. Significant advantages are: possibility to change hydrogels selectivitity to certain ions, possibility to control hydrogels sorption rate to individual ions, relatively fast speed of hydrogels regeneration and ions separation, high selectivity of ion separation.
2. Jumadilov Talkybek Kozhataevich, Himersen Huangul, Kaldayeva Saltanat Serikovna, Kondaurov Ruslan Gennadievich. Features of Electrochemical and Conformational Behavior of Intergel System Based on Polyacrylic Acid and Poly-4-Vinylpyridine Hydrogels in an Aqueous Medium // Journal of Materials Science and Engineering B. – Vol. 4 (6). – 2014. – P. 147-151.
3. Jumadilov T.K., Abilov Zh.A., Kaldayeva S.S., Himersen H., Kondaurov R.G. Ionic equillibrium and conformational state in intergel system based on polyacrylic acid and poly-4-vinylpyridine hydrogels // Journal of Chemical Engineering and Chemistry Research. – Vol. 1 (4). – 2014. – P. 253- 261.
Jumadilov T.K., Abilov Zh.A., Kondaurov R.G. INTERGEL SYSTEMS IN RECOVERY OF PRECIOUS AND RARE EARTH METALS. International Journal Of Applied And Fundamental Research. – 2015. – № 1 –
URL: www.science-sd.com/460-24777 (24.04.2025).









 PDF
PDF