About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
Abstract. The article presents the results of application of the high molecular weight polyacrylamide flocculants of various grades for wastewater purification of industrial enterprises of various spheres of activity from cadmium ions. It describes the main characteristics of one of the most effective physical and chemical methods of purification of industrial wastewater with application of polyacrylamide flocculants, and the main classes of reagents. The action mechanism of substances is justified. The results of purification of wastewaters model solutions of industrial enterprises, which were obtained by applying the method of fractional flocculation, are presented. The results show the effectiveness of this method of wastewater purification of industrial enterprises, suggesting the possibility of its use for studies on wastewater purification from heavy metals such as cadmium.
Keywords: flocculant, purification, waste water, the method of fractional flocculationi, heavy metals, cadmium.
1. Introduction
The potential danger of environmental pollution by heavy metals is one of the most serious environmental problems at present. Severe toxic elements are among the most common pollutants that impact the most harmful effect on the human body and biological objects. There are many different ways of penetrating of this class of substances into water such as the waste water of chemical, chemical-pharmaceutical, metallurgy, metalworking, automotive, instrument-making and other industries.
Cadmium has become one of the priority pollutants among toxic elements due to its wide application in various fields of human activity and large amount of cadmium environmental pollution, respectively. Cadmium and lead exercise their toxic effect at very low concentrations what increases their harmful influence on the environment. The compounds of these metals containing in water contribute to water pollution. The metals are present in water in the form of dissolved or suspended particles and as a part of organo-mineral complexes. On entering the water, metals deposit and then accumulate in sediments. In addition, they impact their harmful effect on the soil when metals are added to it as a part of fertilizers. After contaminating soils they penetrate into plant organisms, and ultimately, into animals and humans. After absorption into the blood soluble components of these metals affect the central nervous system, kidneys and liver, and disrupt calcium and phosphorus metabolism. Chronic poisoning causes anemia and bone destruction. Therefore, removal of heavy metals such as cadmium from the environmental objects is one of the most important tasks of modern chemistry and ecology.
The work of any industrial enterprise can’t be imagined without using huge amounts of water thanks to its main role in all technical processes, and it is the primary component of almost any products. That fact requires continuous improvement of wastewater purification. The choice of method of purification depends on several parameters, such as sanitary requirements for the quality of wastewater, the type of feedstock, the production volume, and many others.
Wastewaters typically contain easily decomposed organic substances, bacteria, heavy metals, dyes, etc. Their temperature ranges from 18 to 20°C. As a rule, their pre- purification includes the mechanical cleaning with the use of lattices, sump, grease trap and sand catchers. Further purification involves application of different groups of methods, such as biological, chemical or physico-chemical. One of the most frequently used methods of purification using polyacrylamide flocculant for sewage cleaning of industrial enterprises is described in this article (up to 97-98% of colloidal and finely impurities).
2. Brief description of recent work in the area described
The main point of the considered coagulation-flocculation method of purification consists of the process of water treatment by coagulants, hydrolysis and the reaction of the hydrolysis products with colloidal or coarse contaminants, that finishes with the formation of flakes [1-4]. To speed up and improve the capacity of the purification of water it is necessary to use in addition to the coagulants some high-molecular substances that are called flocculants. These agents promote the expansion of the optimal areas of coagulation at different parameter values (for instance, temperature and acidity), increase the density and strength of the resulting flakes, enhance the reliability and capacity of purification facilities and reduce the consumption of coagulants. The described above positive sides of using the flocculants improve the quality of purified water.
Sometimes the choice of the using flocculant type is determined by the charge of disperse particle drain and the nature of the charge carriers. It should be noted that the purification process depends on a large number of factors such as class and concentration of pollutants, temperature, acidity of the environment, the type of surfactants and its concentration. Taking into account the previous information we can make a conclusion about the high purification efficiency of the described method [7].
The flocculants include natural and synthetic water-soluble organic polymers. In our country poliakrilamide and its compounds as well as an active silicic acid have traditionally been used in various types of industries. Significant changes in the flocculants market occurred in recent decades. At that time many enterprises discover the imported water purification flocculants of US, Japan, Britain, Germany, France, Finland firms [1-4].
All flocculants can be characterized by various types of macromolecules structures: linear, chain and branched. The majority of substances have a linear structure of macromolecules. The polymer macromolecules made up of a large number of groups (units) interconnected by the forces of chemical affinity. These units can be either homogeneous (homopolymers) or heterogeneous (copolymers). However the number of units of the macromolecules (or degree of polymerization) has the value of 250-70000, the total length of the molecular chain is about 7,5·102-110·102 nm, and molecular weight is in the range of 1·104-1,5·107.
Due to some economic reasons aluminum sulfates (AS) and iron chloride were used for the purification of industrial wastewaters of different origin in our country. Lime was used to adjust the acidity of the environment; and polyacrylamide was put for increasing the size of flakes. The flakes are positively charged in weakly acidic environment, but they are negatively charged in alkaline.
When dissolved in the wastewaters flocculating agents may be ionized or non-ionized. These components can be called soluble polyelectrolytes. Depending on the composition of the polar groups flocculants can be divided into several classes.
Non-ionic flocculantsn are polymers that contain non-ionic groups such as -ОН, >СО (for example, starch, hydroxyethyl cellulose, polyvinyl alcohol, polyacrylonitrile, etc.).
Anionic flocculants are polymers that consist of anionic groups like -СООН, -SO3H, -OSO3H (for example, active silicic acid, sodium polyacrylate, sodium alginate, lignosulphonates etc.). To obtain the anionic flocculant alkaline hydrolysis of polyacrylamide is widely used .
Cationic flocculants are polymers containing cationic groups as -NH2, =NH (for example, polyethylene, copolymers of vinylpyridine, ВА-2, ВА-102, ВА-212, and others.).
Polyethyleneimine (PEI), that is obtained by polymerizing ethyleneimine in the presence of initiating agents, is one of the most effective modern cationic flocculants which is used to clean water.
Amphoteric flocculants are polymers which contain in its molecular structure both anionic and cationic groups (polyacrylamide, and other proteins.).
As part of the practical analysis flocculants with a different sign and magnitude of the charge can be used, but a mechanism of coagulated dirt flocculation is more complex at these conditions and the influence of other factors takes place.
The technology of the application of the organic flocculants has a number of advantages, such as elimination of secondary pollution of water by coagulant hydrolysis products, reducing of the corrosion activity of water, reducing of the amount of produced precipitate, as well as enhancing its ability to dehydration. Flocculants can also be used as efficient precipitants of dissolved ionic contaminants such as surfactants. But in practice the effect of precipitation of soluble organic contaminants is shown in enlargement of suspended impurities of wastewaters.
The choice of the type of flocculants and the efficiency of purification of wastewaters containing dissolved organic impurities depends on their nature and concentration. It should be noted that during the process of flocculation the forming flakes are larger than at another process. During the precipitation of particles for any type of production it is important to take into consideration the main states of La Mer flocculation theory (also known as the sedimentation of particles), that describes the bridge mechanism of flocculation (Fig. 1) [5].
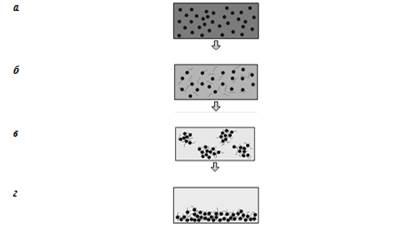
Fig. 1. The basic scheme of binding the dispersion particles by flocculant macromolecules: a – before adding the flocculant, b – after the addition of a flocculant, c – mixing and agglomeration of the particles, d – after the formation of a precipitate
The most commonly used synthetic organic polymers find their application at different types of industries such as coal, mining, petroleum, chemical, pulp and paper, textile, microbiological, etc.
The flocculation ability depends on many factors, including the nature and concentration of the polymer, molecular weight, chemical composition and hydrodynamic macromolecular size, concentration of the disperse phase and composition of the dispersion environment.
In most cases considered agents have a higher efficiency than the original components. Their use simplifies purification technology, expands the area of their effective application and solves the problem of disposal of highly contaminated sewage.
Flocculation theory was developed in the period of 1950-1964 by the French scientist La Mer. In accordance with this theory the flocculation process involves two steps [6]:
1. Some fragments of polymer molecules are adsorbed to the surface of suspended and colloidal particles and occupy a part of that surface (Fig. 2).
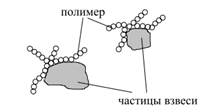
Fig. 2. Scheme of the polymer adsorption on the suspended particles
The entire surface of the particles is considered as 1, the part that polymers occupy – θ; the remaining part – (1-θ).
2. At the second step the secondary adsorption takes place when the remaining free fragments of the polymer molecules are fixed on the surface of other particles, and they bind the polymer particles by linking bridges (Fig. 3). This is the actual process of flocculation.
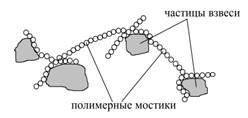
Fig. 3. The formation of polymer bridges during the flocculation
The La Mer rate of flocculation process may be determined according to the following equation:
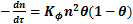 , (1)
, (1)
where n – the number of suspended and colloidal particles per unit volume of water (number concentration); τ – time; Kf – factor that shows the conditions of convergence of particles (distance and speed).
Besides the coagulation rate can also be calculated according to the expression:
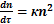 .(2)
.(2)
Obviously, the expression for the flocculation rate differs from the rate of coagulation due to the presence of multiplication expression θ (1-θ), which is a characteristic of flocculation processes, and it determines the probability of a free part of one particle that is located around the polymer molecule adsorbed on the surface of another particle.
Furthermore, the speed of flocculation θ reaches maximum at θ = 0,5. The value of θ depends on the ratio between the surface area of the suspended particle and the polymer dose. The higher dose of the polymer, the greater proportion of the surface of the particles it covers. It is evident that the flocculation is possible only at a certain ratio between the concentration of the suspended particles and the polymer dose:
θ=f(DФ),(3)
DФ=f(n,r),(4)
where dF – flocculation dose; r – size of suspended particles.
The presence of huge adsorbed molecules on the particles surface leads to the flocculation process begins at greater distances than coagulation; besides motion and particle rotation increases the probability of their collision. The increase of the action range and the probability of collision leads to the fact that the flocculation rate is higher than the rate of normal coagulation.
The size of the resulting stable flakes is determined by the following expression:
R=Kn2θ2(1-θ)2 , (5)
where K – const nt that takes into account the intensity of mixing and the bond strength between the polymer and the particle.
Flakes size reaches maximum at θ = 0,5, that means that the best flocculation process occurs if the polymer molecule covers a half of the surface of suspended and colloidal particles.
3. Experimental
Purpose. The main aim of the study is an improvement of the flocculation purification of model solutions of industrial wastewater (WW) and precipitate purification conditions that will enable to made a targeted and reasonable choice of the most effective flocculant, as well as in subsequent studies consider the probability of purification of model solutions of wastewater from inorganic and organic substances (for example, heavy metals, and organic remainders).
Instruments and reagents. Standard solutions of Cd salt (1,0 g/l); distilled water; flocculants of a class AK-631 (A-155, CP-540, N-150) (TU 6-02-00209912-41-94; manufacturer – FSUE «Saratov Scientific Research Institute of Polymers" Ltd. "Gel-Service", Saratov); photoelectrocolorimeter PE5400V; centrifuge; laboratory equipment; stopwatch; solution of lime milk.
Preparation of the flocculant (class AK-631) solution. 0,1 g of grain flocculant is weighed on the technical scales. Then it is dissolved in 100 ml of distilled water with constant stirring with a glass rod. After dissolution of all flocculant particles the glass with the prepared solution is placed on a magnetic stirrer and it is stirred for 1,0 hour. The gelatinous mass should be formed at end of the process [7, 8].
The flocculation of model wastewater (WW) by the flocculant (class AK-631) solution. Model WW is poured into four measuring cylinder of 500 ml (the first cylinder – 0 ml (control); the second – 0,1 ml; third – 0,5 ml; the fourth – 1,0 ml.), and the solution of lime milk is added in small portions to it for getting a certain value of pH. All four cylinders are stoppered and mixed thoroughly by shaking. Then the rate of precipitate sedimentation and the time of full sedimentation are determined in each cylinder. After sedimentation of the precipitate the mixture is left for some hours for sediment compaction. Then water samples are collected in flasks for heavy metal ions analysis of WW.
The purification efficiency is calculated from the following equation:
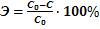 ,(6)
,(6)
where Э – purification efficiency,%; C0 – initial concentration, mol/dm3; C – residual concentration, mol/dm3.
4. Result and Discussion
The calculation results of water purification efficiency from cadmium ions by fractional flocculation are shown in table 1.
Table 1
The efficiency of water purification from cadmium ions by fractional flocculation of flocculant (class AK-631) [8]
|
Flocculant |
Initial metal concentration in model WW·104 mol/dm3 |
The amount of flocculant solution, ml |
Purification efficiency, % |
|
А-155 |
3.5 |
0,1 |
71,83±7,00 |
|
0,5 |
71,83±7,00 |
||
|
1,0 |
70,75±7,00 |
||
|
КП-540 |
3,5 |
0,1 |
76,15±7,60 |
|
0,5 |
79,88±8,00 |
||
|
1,0 |
80,17±8,00 |
||
|
Н-150 |
3,5 |
0,1 |
71,21±7,00 |
|
0,5 |
71,84±7,00 |
||
|
1,0 |
74,43±7,40 |
5. Conclusions
Taking into account the previous calculations we can conclude that the purification efficiency by using macromolecular polyacrylamide flocculant has the value of 70 to 80%, so we can say about the high effectiveness of the considered WW purification method, the relevance of the problem and the possibility of its application for model wastewater purification studies not only from heavy metals and inorganic substances, but from organic substances.
Based on the information above we can draw the following conclusion: the mass using of organic flocculants and coagulants at all stages of the technological processes for purification of industrial wastewater will improve efficiency, reliability and stability of sewage purification plants while reducing their overall performance and minimizing capital and operating costs, improve water quality of surface water sources by reducing the release of harmful substances with wastewaters.
2. Terekhova E.L. Intensification of wastewater purification from surfactant / «05.23.04» Water supply, sew-erage, building systems of protection of water resources». – Dis. cand. tehn. Sciences. – Khabarovsk. – 2004. – 178 p.
3. Gandurina L.V. Organic flocculants in technology of purification of natural and industrial wastewater and precipitate treatment // Ing. secu. objects p.: actual situation review. inf. – M.: VNIINTPI. – 2000. – Issue 2. – 59.
4. Frolov Yu.G. Course of Colloid Chemistry. Surface phenomena and disperse systems. – Ed. 2nd Revised. and ext. – M.: Chemistry. – 1988. – 464s.
5. Kurenkov V.F. Polyacrylamide flocculants // Sarov educational journal. – 1997. – № 7. – S. 57-63.
6. Kulikov N.I., Naimanov A.Y., Omelchenko N.P., Chernyshov V.N. Theoretical basis of water purification / Lectures. – Donb. nat. Acad. c Islands and architecture. – Makiyivka. – 2009. – 297 p.
7. Shachneva E.Yu. Physical chemistry of adsorption of flocculants and synthetic surfactants on sorbent SV-1-A: Dis. cand. Chem. Sciences: 02.00.04. – Makhachkala. – 2011. – 139s.
8. Industrial Ecology Basics: guidelines for laboratory works on discipline «Industrial Ecology Basics» / comp. M.V. Buzaeva, V.V. Semenov, P.O. Osipov. – Ulyanovsk: UlSTU. – 2008. – 31 p.
Shachneva E. SELECTIVE USE OF HIGH MOLECULAR WEIGHT POLYACRYAMIDE FLOCCULANTS OF DIFFERENT CLASSES FOR WASTEWATER PURIFICATION OF INDUSTRIAL ENTERPRISES FROM IONS OF CADMIUM . International Journal Of Applied And Fundamental Research. – 2017. – № 3 –
URL: www.science-sd.com/471-25346 (03.02.2026).











 PDF
PDF