About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
 PDF
p. 3-4
PDF
p. 3-4
As of today surfactants has wide usage scope in many economic sectors: detergents, flotation reagents, emulsion and foam stabilizers, dispersants of minerals, antistatic agents, corrosion inhibitors, demulsification agents, etc.
The study objective is producing of surfactants from available and having the wide usage scope substances for organic synthesis: from fatty acids of sylvic oil and from ethanolamines. Characteristics of synthesized surfactants are also studying.
Diethanol amine was used for production of non-ionic surfactant.
With equimolar ratio of components the reaction is processed according to the following scheme:
RCOOH + NH(C2H4OH)2 → RC(O)N(CH2CH2OH)2 + H2O,
where R is the rest of fatty acid.
Sylvic acid diethanol amide (SADA) was produced. Trietanolamine was used for production of cationic surfactant.
With equimolar ratio of components the reaction is processed according to the following scheme:
RCOOH + N(C2H4OH)3 → R(O)CОСН2СН2N(CH2CH2OH)2 + H2O,
where R is the rest of fatty acid.
Sylvic acid trietanolamine (SAT) was produced. It is a cationic surfactant.
Reaction control was performed by changing the acid number. At the end of process it was equal to 5,0-5,2 mg КОН/g for SADA and 5,5-5,8 mg КОН/g for SAT.
The duration of production process for SADA and SAT is 4-5 hours. Time & temperature mode of synthesis of SADA is presented at the Figure. Parameters of synthesis for SAT are the same as presented at the Figure.
The synthesized products at indoor temperature are brown viscous aggregations. The structure of produced surfactant is corroborated by the findings of IR-spectroscopy.
During this study IR-spectrums were created in liquid film between the KBr plates using the instrument SPECORD 75 IR in the range 3800-700 cm-1. These spectrums were interpreted: see [1] for additional information.
Thereby the findings of IR-spectroscopy corroborate the presence of characteristic functional groups in synthesized surfactant. The produced non-ionic surfactant is characterized by first and second amide lanes. The synthesized cationic surfactant contains hydroxyl, carboxylic, ester groups.
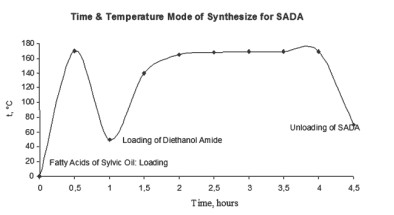
Time & Temperature Mode of Synthesize for SADA
Colloid-chemical characteristics of SADA and SAT were studied. Emulsifying, foam-production and other characteristics were estimated. Temperature of turbid formation for water solutions of non-ionic surfactants having the concentration 1 g/dm3 was determined. Experimental and computed data relevant to study of characteristics of SADA and SAT solutions are presented
in Table.
Characteristics of SADA and SAT Water Solutions
|
Characteristic |
Value |
|
|
SADA |
SAT |
|
|
HLB |
12,625 |
11,68 |
|
CCMF, mole/dm3 |
3,7-3,8·10-3 |
3,1-3,2·10-3 |
|
Temperature of turbid formation for water solution with concentration 1,0 g/dm3, °C |
82,0 |
- |
|
Foam's ratio |
0,048 |
0,030 |
|
Stability of emulsion pending 2 hours |
Is stable |
|
|
pH of 1 % water solution |
6,5-7,0 |
7-8 |
Degree of adsorption on the surface for surfactants depends on structure of surfactants' molecules. Ratio between hydrophilic and hydrophobic shares (HLB) is the quantitative characteristic of surfactant's usage scope.
Turbid formation temperature (turbid point), HLB value and surfactant's usage scope are interrelated.
According to computed for SADA and SAT values of HLB the synthesized surfactants can be used as emulsifying agents «oil in water». These surfactants are easy soluble in water transparent dispersions. See [2] for additional information.
Reduce of surface tension is the basic estimation criterion of surfactant's action. See [3] for additional information. During this study the surface tension was measured according to tear of the ring procedure (for solutions). Construction of graphs for dependency between values of surface tension and values of concentration allowed determining the point of CCMF - the critical concentration of micelle formation. CCMF of SADA is equal to 3,7-3,8·10-3 mole/dm3, CCMF of SAT - 3,1-3,2·10-3 mole/dm3.
Turbid point has the practical meaning: stability of emulsion (containing surfactant as emulsifying agent) depends on temperature, at which emulsion was created. Temperature mode for creation of stable emulsion is determined by surfactant's turbid point. Temperature of creation for emulsion must not exceed the turbid point of surfactant's solution.
Average temperature of turbid point for synthesized SADA was equal to 82 °C.
Ability to create foam is characterized by foam's ratio - ratio between volume of foam and volume of surfactant's solution. The foam's ratio was equal to 0,048 for SADA and was equal to 0,030 for SAT. These values are small - this corroborates presence of non-ionic and cationic characteristics in synthesized products. These products are also characterized by relatively small foam producing ability.
The foam stabilizing ability is characterized by kinetic stability pending required period of time.
It is known, that stabilizing and emulsifying activities of surfactants are closely linked. They determine aggregative stability of emulsion - it is usually characterized by duration of existence (lifetime) for separate drops contacting each other or contacting with interfacial surface. This is also known as emulsion's delamination rate. The stability of produced emulsions was tested. The emulsions were diluted up to 0,5 % of concentration and were shook energetically. No delamination was observed pending 2 + hours, so, the produced emulsions are stable.
Existence of various tensions between dispersed phase (oil) and dispersive medium (water) explains stabilizing activity of surfactant [4].
Presence of surface active characteristics (foam producing, wetting, emulsifying, stabilizing) for synthesized SADA and SAT is corroborated by the study findings.
1. Silverstein R. Spectrometric Identification of Organic Compounds / R. Silverstein, G. Bassler, T. Morril. - Moscow: «Mir» publishing house, 1977. - 592 p.
2. Lange K.R. Surface-Active Agents: Synthesis, Characteristics, Analysis and Usage / K.R. Lange; Edited by L.P. Zaichenko. - St. Petersburg: «Professia» publishing house, 2004. - 240 p.
3. Lutfullina G.G. Study of characteristics of synthesized non-ionic surfactants // Bulletin of Kazan State Technological University. - Kazan: KSTU, 2011 year - Vol. 6. - P. 44-47.
4. Sherman F. Emulsions. - Leningrad: «Chemistry» publishing house, 1927. - 448 p.
Silverstein, G. Bassler, T. Morril.
Lutfullina G.G., Abdullin I.S. BASED ON FATTY ACIDS OF SYLVIC OIL SURFACTANTS: SYNTHESIS AND STUDY OF SURFACE ACTIVE CHARACTERISTICS. International Journal Of Applied And Fundamental Research. – 2012. – № 1 –
URL: www.science-sd.com/450-24000 (11.02.2026).










