About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
Introduction
One of the barriers to the widespread use of whey for food is its high relative ash, two times higher than for milk. For this reason, whey concentrate, unlike milk, have a bitter-salty taste. This problem is solved demineralization - Purified serum from dissolved salts, particularly the sodium salt. The process of demineralization of whey expanding the scope of its use in the food industry, received demineralized whey concentrates have a clean sweet taste and are used to produce a wide range of healthy foods.
Technological operation of demineralization of whey used in the technology of functional dairy products with low residual allergenicity. It is aimed at removing one of the serum and divalent metals, and reduced active acidity.
Desalting solutions can be carried out in different ways: ion exchange, electrodialysis, nanofiltration or reverse osmosis.
As a method of desalination, reverse osmosis is used in large-scale desalination of sea and ocean waters, but it is not applicable to the whey in that capacity.
The purpose of the nanofiltration - to filter out part of the electrolyte, while the remaining components of the concentrate treated solution without significant losses. In the future, as the technology of manufacturing membranes, nanofiltration can be the most interesting membrane process for the dairy industry, which combines the principle of concentration and demineralization.
The aim is to study the process of demineralization of whey by various methods.
Materials and Methods
Electrodialysis - membrane separation process in which ions of dissolved substances are transported across the membrane in an electric field. Driving force is the gradient of the electric potential. In multiple electrodialyzer alternates large number (several hundred) of cation-exchange and anion-exchange membranes located between two electrodes (Fig. 1).
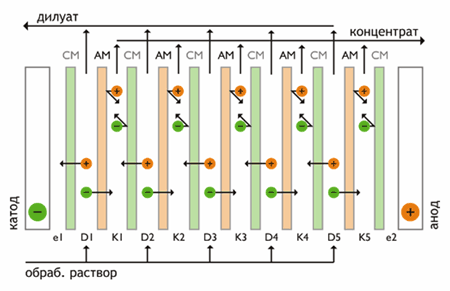
Figure 1 - Scheme of electrodialysis
Electric current moves cations from a stock solution in concentrate flow through the cation exchange membrane disposed on the cathode side. Cations are retained in this flow anion exchange membrane on the cathode side. The direction of movement is the opposite of anions. They moved to the concentrate flow through the anion exchange membrane. The anode side anions retained in the concentrate stream cation exchange membrane. Thus, the overall outcome of the process is to increase the concentration of ions in alternating cells while reducing their concentration in other cameras. The electrodes is the process of electrolysis.
In multi-unit unavoidable overhead electricity due to this process, distributed on a large number of cameras. Therefore, based on the unit of production, these costs are minimized.
In classic desalting whey using cation-and anion-exchange membranes are faced with two potential obstacles caused by clogging of membranes: deposition of poorly soluble calcium salts and the deposition on the surface of the anion exchange membrane protein fractions (mainly amino acids). Many components of the denatured proteins are large, negatively charged ions that move within the membrane package under the influence of electric current. These particles are too large to be able to pass through the anion-exchange membranes, and they are deposited in a thin layer of cells on the surface of the serum anion-exchange membranes. Sediments with the membrane surface can be removed changing polarity of the current, but this does not always lead to the desired results.
If the membrane is continuously operated at current densities above 20-25 mA/cm2 proteinopodobnye layers can settle permanently, causing rapid deterioration and even the disappearance of the anion exchange membrane properties. Under typical operating conditions with normal fresh serum in normal current densities (10 - 25 mA/cm2) deposition proteinopodobnyh factions usually leads to clogging of the membranes and the beginning of degradation after a period ranging from two weeks to several months. Electrodialysis good for demineralization of highly saline solutions with high electrical conductivity. He is easily automated and does not require for its operation of large amounts of chemicals. However, at low concentrations of minerals, when the electrical treated solution tends to zero, the efficiency of electrodialysis drops.
In practice, ion exchange and electrodialysis, as two direct method of desalination, complement each other. If necessary, finish sweep of mineral impurities commonly used ion exchange. The advantage of ion exchange is the ability to virtually complete cleaning solutions from minerals.
Ion exchange method is industrially direct desalination of mineralized solutions. Ion exchange is carried out in columns with ion-exchange resins (Fig. 2).
Figure 2 - ion-exchange column
They are natural or synthetic electrolytes, structurally composed of a rigid framework (matrix) and functional groups, rather firmly connected by chemical bonds. Depending on the charge on the cation exchange resins are classified (negative charge), anion (positive charge) and ampholytes containing cations in the (H +) and anions (OH-). The principle of this method is based on the mobile ion exchange stationary phase and like-charged ions from a flowing solution.
Ion exchange is used in the dairy industry in obtaining lactic acid for demineralization of whey, remove radioactive elements from raw milk, high heat stability of milk intended for sterilization to reduce its acidity. Whey is passed first through a cation exchange resin and then through anion exchanger. Cation binds cations present in the whey minerals, highlighting the appropriate acid anions are bound anion.
After passing through the two columns with ion exchangers efficiency demineralization of whey, depending on its type is 90 - 99%. For the production of certain products less desirable (50 - 60%) degree of demineralization. In these cases, demineralized whey mixed automatically in the appropriate proportions (for pH regulation) with untreated.
Exchangers shall be automatically regenerated after each run. To carry out this process to recommend the following procedure for the preparation of adsorbents for regeneration and storage: pre-wash water treatment disinfectant detergent, rinse with cold water, regeneration solution ugleammoniynoy salt, rinse with cold water. Regenerated resin stored under water before the next process of demineralization of serum.
Cation exchange resin is regenerated with HC1 on the counterflow principle. When compared with the once-through technology countercurrent ion exchange resin regeneration system provides reduced consumption of reagents for regeneration, improving the quality of the treated water and the reduction of consumption for own needs. To regenerate the anion resin solutions are used Na2CO3 or NH4OH.
Optimizing manufacturing operation whey demineralization performed as electrodialysis installation and by ion exchange.
The main equipment for the process of demineralization of whey is a laboratory electrodialysis, the concept of which is shown in Figure 3.
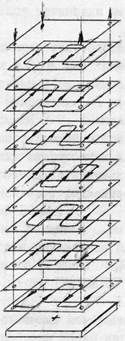
Figure 3 - Schematic diagram of the laboratory electrodialysis
Electrodialysis consists of nine horizontal chambers with sequential hydraulic connection, and two electrode chambers. The device incorporates heterogeneous ion-exchange membranes MK-40 and MA-40, frames and labyrinth type seals, made of viniplastovoy calendered film electrodes made of stainless steel. Electrode chambers were separated from the working rubber gaskets labyrinth type 30 mm thick. The thickness of the chambers in electrodialyzer 1.2 mm working area of 0.224 m2 membrane, desalination path length 3.4 m fundamentally laboratory electrodialysis apparatus complies electrodialysis plants, which are used in the dairy industry for the regulation of the whey.
For the process of demineralization laboratory electrodialysis include a DC circuit, the source of which is stabilized rectifier collected on the bridge circuit of the semiconductor diodes, voltage regulation is performed by a laboratory autotransformer LATR-1. Monitoring current exercise voltammeter M 2044 (Figure 4).
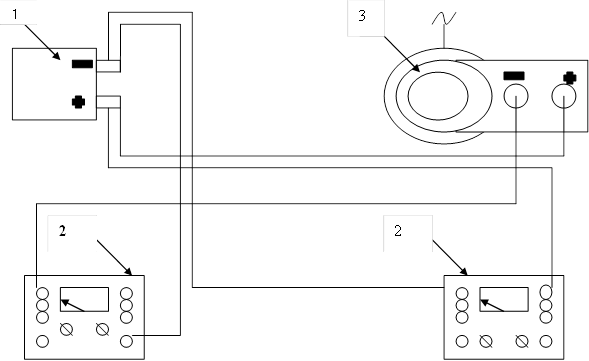
Figure 4 - Circuit diagram electrodialysis installation: 1-electrodialysis, 2 - voltammeter, 3 - LATR.
Cation membrane MK-40 are made from a strongly acidic cation exchanger KU-2, the anion exchange membrane izoporistye fabricated using strong-anion-17. Physico-chemical and mechanical properties of membranes depends on the technology and the slightly vary from batch to batch.
In order to improve the physical and chemical properties of the membranes prior to use spend their pre-deployment training. For this, the surface membranes were treated with carbon tetrachloride to remove Shrove film. 10 minutes after the escape of solvent from the surface of the membranes were immersed in ethanol, which held 6 hours after alcohol treatment cation and anion exchange membranes were subjected to the separate swelling for two days in a concentrated solution of sodium chloride. Then they kept two days in distilled water.
Next, the membrane was treated with 3N cation. sodium hydroxide solution during the day, and then 3N. hydrochloric acid solution, after which they were washed until neutral to phenolphthalein. Anion exchange membrane was treated first 3N. hydrochloric acid, followed by 3N. sodium hydroxide solution and then washed with distilled water until neutral to methyl orange. Membranes were stored in the working solution. Swelling of the membrane separated viniplastovymi corrugated gaskets to ensure free access to the solution.
In practice, the ED-treated serum try to stick to the following rule: the voltage of the chamber is 2, the maximum of 3V. This is due to the fact that the very high currents, especially in the final stage of ED treatment, when the ash content of the product is low, the growing concentration at the membrane surface changes in cells desalination. In this case, the efficiency of the process is significantly reduced. In predmembrannyh areas, and, in the first place at the surface membrane anionoselektivnyh begins hydrolysis of water molecules into ions H + and OH-. Treated product acidic, since the ions OH-output through the membrane, and H + ions enter the product.
Further increase in tension on the membrane frames in concentration polarization increasingly large part of the current begins to be spent on the involuntary transfer of H + and OH-. Concomitant decrease in serum pH increases the risk of coagulation of whey proteins. Thus, if a laboratory dialyzer consists of 9 cameras, the voltage per cell 3.2 V, the work can be performed at the dialyzer 18 - 27 V.
For the process of demineralization feedstock was used cheese and cottage cheese whey, raw material indices are shown in Table 1.3. Demineralization process was conducted in a laboratory setting electrodialysis EDU at room temperature for one hour. To control the level of demineralization of whey the dependence of the electrical conductivity of the whey mineral content (ash) (Figure 5). This technique enabled the technological instructions for the production of demineralized whey as an operational method to control the primary process variable - the ash content of serum.
Table 1 - Physical and chemical characteristics of the processed whey
|
raw |
Mass% |
acidity |
|||||
|
solids |
including% |
titrated, ° T |
Active |
||||
|
squirrel |
fat |
lactose |
ash |
||||
|
whey cheese |
6,47±0,2 |
0,65±0,3 |
0,3±0,02 |
5,0±0,01 |
0,5±0,01 |
18±0,5 |
5,8±0,1 |
|
whey curd |
5,83±0,2 |
0,53±0,3 |
0,3±0,02 |
4,4±0, |
0,6±0,01 |
75±0,5 |
4,7±0,1 |
Results and discussion
Physico-chemical characteristics of cheese and curd whey depends on the mode of production of cheese and cottage cheese, and differ in acidity and lactose and protein.
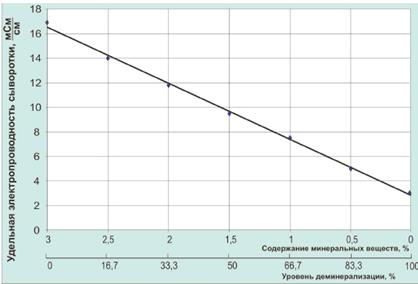
Figure 5 - The dependence of electrical conductivity of the serum level of demineralization.
As seen in Figure 5 decrease mineral content decreases conductivity of serum, and the level of demineralization increases. The electrical conductivity of the original whey 14.8 mS • cm-1 curd - 16.8 mS • cm-1, and after the process of electrodialysis treatment - 9.0 mS • cm-1 and 9.6 mS • cm-1, respectively, which corresponds to 50% degree of demineralization. Level control of demineralization of whey during electrodialysis can also be done on the dynamics of change in titratable acidity (Figure 6).
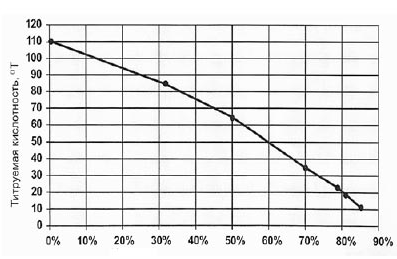
Figure 6 - The relationship between the level of titratable acidity demineralization
Table 2 shows the physical and chemical properties of cheese and cheese whey after the electrodialysis process.
Table 2 - Physical and chemical serum after ED treatment
|
raw |
Mass% |
acidity |
|||||
|
solids |
including% |
titrated, ° T |
Activeя, |
||||
|
squirrel |
fat |
lactose |
ash |
||||
|
whey cheese |
6,04±0,2 |
0,64±0,3 |
0,3±0,02 |
4,9±0,01 |
0,2±0,01 |
12±0,5 |
6,2±0,1 |
|
whey curd |
5,42±0,2 |
0,52±0,3 |
0,3±0,02 |
4,3±0,01 |
0,3±0,01 |
33±0,5 |
6,0±0,1 |
After the electrodialysis, a decrease of mass fraction of solids, due to the removal of a number of salts of the serum during demineralization, a decline of titratable acidity - the removal of chloride ions and anions of inorganic acids. The mass fractions of whey proteins, fat and lactose changed slightly.
After the process of electrodialysis in cheese and cheese whey content was determined one and divalent metals by capillary electrophoresis. The results are reported in Table 3.
Table 3 - The results of the cationic composition of the serum after ED treatment by capillary electrophoresis
|
sample Number |
name cation |
Cation content, mg/dm3 |
|
whey |
K |
984,912±98,491 |
|
Na |
938,999±93,900 |
|
|
Mg |
280,641±28,064 |
|
|
Ca |
695,331±69,533 |
|
|
demineralized whey |
K |
465,763±46, 576 |
|
Na |
403,285±40,328 |
|
|
Mg |
136,477±13,647 |
|
|
Ca |
349,375±34,937 |
The study showed that during the electrodialysis process whey is reduction of monovalent and divalent metals on average by 50%.
To investigate the process of demineralization by ion exchange using high kationonitnye anionite resp resin and domestic production, developed in the Institute of Chemical Technology. This styrene-DVB resins: highly acidic sulfonic CG-2H-8 in the ammonium form and strongly basic anion exchange resin AV-17-8. Experiments on whey demineralization by ion exchange was carried out using ion-exchange column (Figure 7), one of the columns filled with cation exchange resin, and the second - anion.
Figure 7 - The ion exchange column
After the preparation of ion-exchange resins and wash water source serum passed first through a cation, then through an anion exchange resin. The electrical conductivity of the serum decreased from 14.8 index mS • cm-1 to 80,6 • 10-3 mS • cm-1, which corresponds to 90% level of demineralization of whey. Cation content in the sample was low (Table 4).
Table 4 - The results of the cationic composition of the serum by capillary electrophoresis after passing through the ion exchange resin
|
sample Number |
name cation |
Cation content, mg/dm3 |
|
whey |
K |
984,912±98,491 |
|
Na |
938,999±93,900 |
|
|
Mg |
280,641±28,064 |
|
|
Ca |
695,331±69,533 |
|
|
demineralized whey |
K |
3,622±0,362 |
|
Na |
17,951±1,795 |
|
|
Mg |
7,411±0,741 |
|
|
Ca |
11,454±1,145 |
Analysis of experimental data confirms the conclusion drawn earlier on the basis of the literature review. After passing through the two columns with ion exchangers efficiency demineralization of whey, depending on its type is 90 - 99%, thus there is little loss of serum proteins. The disadvantage of ion exchange is that after saturation resin minerals treated solution is subject to regeneration acids and alkalis. The advantage of ion exchange is the ability to virtually complete cleaning solutions from minerals, low maintenance costs for heat and electricity, the use of cheap and affordable domestic production of ion exchange resins, durability cation and anion exchange resins.
Demineralized whey by ion exchange is less pronounced bitterness due to the removal of minerals, low acidity (this is especially important for sour curd whey in its further use), making it possible to use serum in the production of functional foods with low residual allergenicity.
The work is funded by the Ministry of Education and Science under the federal target program "Research and development on priority directions of scientific-technological complex of Russia for 2007-2013", the state contract № 12.527.11.0008.
Retsnzenty:
Nikolai Kucher, D.Sc.. Professor FGBOU VPO "KemSU";
Tatiana Shevchenko, Professor FGBOU VPO "KemTIPP"
References
1. Tokayev ES, Bazhenov EN, parasite RY Contemporary experience and perspectives of agents in the production of whey protein functional beverages / / dairy industry. - 2007. - № 10. - S. 55-56.
2. RF Patent № 2375910 C1, 20.12.2009.
3. Sinha R., Radha C., Prakash J., Kaul P. Whey protein hydrolysate: Functional properties, nutritional quality and utilization in beverage formulation / / Food Chemistry. - 2007. - Vol. 101. - P. 1484-1491.
Prosekov A.Ju., Ulrih E.V., Budrik V.G., Botina S.G., Agarkova E.Ju. ON WAYS DEMINERALIZATION OF WHEY. International Journal Of Applied And Fundamental Research. – 2013. – № 1 –
URL: www.science-sd.com/452-24049 (11.02.2026).











 PDF
PDF