About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Materials of the conference "EDUCATION AND SCIENCE WITHOUT BORDERS"
Sulfonic acid are valuable raw materials for the synthesis of various organic compounds, and also are used in the production of organic dyes, drugs. In the process sulfoxidation as sulfonating agent applies sulphur dioxide, which is one of the most massive and toxic pollutants of the atmosphere, therefore, the process of sulfoxidation can be used for cleaning of sulfur dioxide.
Optimal conditions of low-temperature reaction of catalytic sulfoxidation aromatic compounds determined on the basis on the systematic study kinetics of the reaction:
ArH + 2SO2 + O2 → ArSO2OH + H2SO4 (1)
Kinetic results describes the equation:
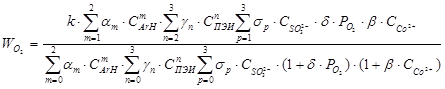
where b - constant formation of binucleate cobalt complex, am, gm, sp, d - constant formation of binucleate cobalt complexes with ArH, PEI, Na2SO3,O2 respectively, k - partial constant of speed.
Kinetic researches suggest the mechanism of the reaction:
In the framework of semiempirical method PM3 [1] calculation the spatial and electronic structure of the main reaction stages.
At the first stage of reaction is oxygenated complex (I). The next phase of the reaction is a complex II. In II molecule sulphurous acid very poorly coordinated ArH, transfer of electron density is ~0.01, and atom O3 is at a distance of ~2,5Å from carbon atoms in the benzene ring. In the limiting reaction stage is activated s-complex (III) there has been a transfer of electron density with undivided electronic pair of sulfur atom to the benzene ring atom.
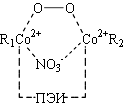
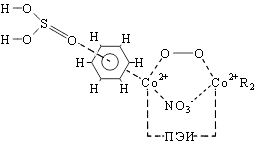
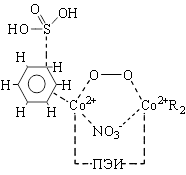
|
a, R1 = ArH; R2 = H2SO3; DE = -140,3 |
a, R3 = H2SO3 |
a, R3 = H2SO3; |
|
b, R1 = ArH; R2 = |
b, R3 = |
b, R3 = |
|
c, R1 = ArH; R2 = |
c, R3 = |
c, R3 = |
|
d, R1 = |
|
|
At the fourth stage of the reaction is activated complex IV with virtual fourth S-O bond. In structures I-III on the ion cobalt concentrates rather negative charge, and on an atom of sulfur SO32- a big positive charge. Formation of a virtual S-O bond, and small activation energy caused, apparently, the influence of electrostatic field inside the complex. In IV charge on the atoms of oxygen molecules have a growing and connection O-O breakes finally, the reaction ends with the formation of a stable complex V. In V is observed jump hydrogen ion with ArH on to free oxygen atom with generation, ОН¯ is.
The possibility of coordinating atom of carbon benzene ring was repeatedly discussed in the literature [2-3]. In oxygenic complex (I) there is transfer of electron density with ArH on ion cobalt atoms and molecules of oxygen, because of this charge on the coordinated carbon atom is significantly increased. Probably, therefore, activated III complex is formed with an activation energy ~4 kkal/mol, which provides the reaction at temperatures ~60°С.
2. Dewar M.J.S., Dieter K.M.// J.Am.Chem.Soc.1986.V.108.P.8075-8076.
3. Коптюг В.А.//ЖВХО им. Д.И. Менделеева, 1976, Т.21, 3, С.247
Emelyanova V.S., T.V. Shakieva, Zh.K. Kaiyrbekov, B. Dosumova, U. Dzhatkambayeva, ZH.K. Myltykbaeva, D.Zh. Mukhitova CATALYTIC SULFOXIDATION OF AROMATIC COMPOUNDS IN THE PRESENCE OF ATTACHED TO THE POLYETHYLENEIMINE OF COBALT IONS.. International Journal Of Applied And Fundamental Research. – 2013. – № 2 –
URL: www.science-sd.com/455-24246 (24.02.2026).











 PDF
PDF