About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Chemical sciences
The plant easily endures a drought, excessive humidity and even a subpolar cold.
Helianthus tuberosus can grow up to four metres in height that’s whyit easily suppresses weeds and consequently does not need chemical processing.
The tubers “forgotten” in the field perfectly endure wintering and by the spring only accumulate useful properties. In general, it is the crop for the lazy farmer.
Helianthus tuberosus draws increasing attention as biopower crop which can be processed into food, medical and technical products. Due to a considerable quantity of carbohydrates in tubers, including inulin (from 15 to 35%) it is used as raw materials for fructose extracting, for preparation of syrups, juice, nectar, fruit candy and flour from which dietary bread is baked, etc. It is possible to receive 9-10 kg of fructose from 100 kg of tubers, whereas from 100 kg of sugar beet – only 4-6 kg. fructose differs by a number of valuable properties. So, bysweet degree it exceeds usual sugar approximately by 70%; dissolves in water better than sugsr; absorbs well moisture from air preventing from hardening the confectionery made out of it; also it is assimilated faster by human body than usual sugar and consequently it is widely used as a treatment of diabetes [1].
Inulin is natural polysaccharide extracted from tubers and roots of some plants. The inulin content is the highest in Helianthus tuberosus. There are a lot of it in chicory, garlic, dandelions and in popular nowadays Echinacea. The modern “cold” extraction technology allows careful allocation of inulin from these plants having kept biological activity.
Inulin influences radically on metabolism. Hydrochloric acid of stomach and intestines enzymes split inulin on separate molecules: fructose and other small fragments which get into blood channel.
Inulin renders immunomodulsting and hepatoprotective action counteracting occurrence of oncological diseases.
Taking medicine containing inulin allows lowering suger level at diabetes and prevents occurrence of diabetes complications (retinopathies, angiopathy and etc.).
Owing to it inulin is applied as a biologically-active additive formedical and preventive food at diabetes of I and II type, including complicated diabetic – angiopathy. In fact almost hundred millions persons suffer from diabetes all over the world.
Pertinently to use inulin at obesity, atherosclerosis, ischemic heart disease, myocardium heart attack, gallstones and nephrolithiasis illnesses, arthritises and osteochondrosis [2,3].
The purpose of our research is the establishment of inulin structure extracted from of Helianthus tuberosus.
Earlier the optimum conditions of inulin allocation and clearing from Helianthus tuberosus were developed and defined by us [4].
Infrared spectrum was taken on a spectrometer “Thermo Electron Corporation” (USA) in KBr; Nuclear magnetic resonance spectra 1H and 13C was registered on device “Jeol” (Japan) (working frequency 400 MHz), specific rotation on polarimeter “Π-161 №811170”. Diffractogram was taken on device “DRON-3” with Cu Kα – radiation at a working mode 35 kW, 20mA and Co Kα 30kW, 20mA. Inulin microstructure was taken by a scanning electronic microscope “JSM-6510 LA”. The inulin molecular weight is defined by a viscometer method.
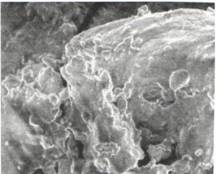
Fig.1. Inulin microstructure from plants Helianthus tuberosus (x 6000 in an electronic microscope)
The inulin microstructure extracted of Helianthus tuberosus showed that it is polymer and specific rotation -30-35 corresponds to the literary data [5]. Molecular weight is 6540-10854 Dalton.
The infrared spectrum of inulin is characterized by presence of a strip’s absorption of valency fluctuations of hydroxyl groups in area 3408,9-3343 cm-1, valency fluctuations of methyl groups in area of 2934,9-2891,8 cm-1. In infrared spectrum there are intensive strips of absorption of deformation fluctuations of hydroxyl groups of 1056,7-1002,4 cm-1 corresponding to valency fluctuations of binding CO and also a strip of absorption of 1718,1 cm-1 to COH valency fluctuations.
The analysis of Helianthus tuberosus has shown that one has high, two have average and at the others have low intensity.
Diffraction lines intensity of Helianthus tuberosus and the extracted substance are characterized by following interplanar distances accordingly: d=3,35; 4,07; 4,97; 5,37; 7,25 A0; and d=3,27; 4,06; 4,08; 4,40; 4,69; 4,73; 4.98; 5,21; 5,41; 7,22A0.
There are 10 reflexes in inulin diffractogram – seven of which are high, two –average and one reflex of crystal strips – low.
Thus, it is shown that diffractograms of Helianthus tuberosus and of inulin considerably differ on interplanar distance and intensity of crystal strips that specifies allocated new structure which differs from Helianthus tuberosus.
Structure allocated polysaccharide of inulin was established on the basis of the given spectra of a nuclear magnetic resonance 1H and 13C. (Fig.2). the spectrum becomes complicated because of the big molecular weight of inulin, consisting of D-fructose residue connected by 1,2-glycosidebindings. NMR spectrum represents wide intensive multiplet signal in the field of 3,25-4.91 m.part. Such character of a signal is caused by chemical protons nonequivalence of fruitpyranose and fruitfuranosyl inulincycle and difficult intermolecular interaction of nucleus. The resonance of protons in one area specifies in a carbphydrate chain which consists of repeating links of fructose residue. However it is possible to distinguish multipletpeaks related to fructosenuclear groups. So chemical shifts in the field of 3,25, 3,40 and 3,57 m.part. are related to methyl groups of fructose. A signal in the field of 3,82 m.part is referred to methylene group of glycosidebinding. Chemical shifts of hydroxyl groups join with the core multiplet in field of 4,91 m.part.
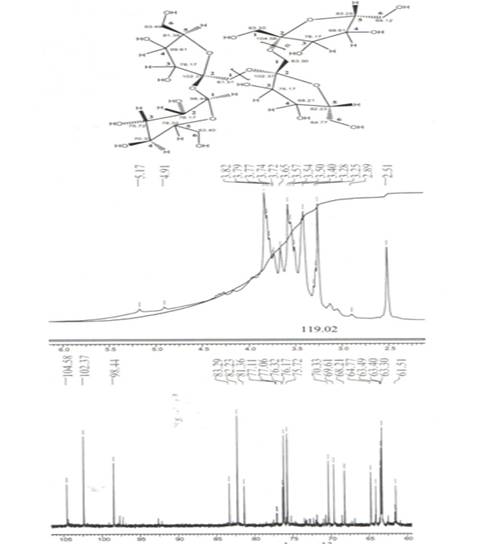
Fig.2.Data of nuclear magnetic resonance spectrum NMR 1H and 13C of inulin.
The nuclear magnetic resonance spectrum 13C represents a set of signals related to carbohydrate atoms of carbon. Values of chemical shifts of carbon atoms are presented in Figure 2. Chemical shifts of cyclic atoms of carbon fruitpyranose and fruitfuranosyl cycles are shown in area δ 68,21-76,32 m.part. atoms of carbon methylene groups S1 and C6 resound in area δ 61,51-64,77 m.part. Chemical shifts of carbon atom C1 of fruitpyranose cycle is shown in area δ 98,44 m.part. Signals in weakly complete areas δ 102,37 and 104,58 m.part. are related to quaternary carbon atoms C2 of fruitfuranosyl cycles.
The obtained data of compound of infrared spectrum, a nuclear magnetic resonance 1H, 13C spectra anddiffractogrammof x-ray diffractionanalysis gives basis to confirm extraction of new natural compound from Helianthus tuberosus structure of which is identical to inulin’s.
2.S.A.Summer, Avtoreferta.dis… Cand. Tech. Sci., Voronezh State agrarian University, Voronezh, 2011. -21pg.
3.M.T.Kisieva, Dissirtation of Candidat of Pharmacy Sciences. Pyatigorsk State Pharmaceutical Academy-Pjatigorsk, 2011.-143pg.
4.Azimbaeva G.E., Butin B.M., Kudaybergenova G.N. Allocation and ways of inulin clearing from Helianthus tuberosus L.//Scientific life. –M: the Science, 2010.-№2. 6-11 pg.
5. Hodzhaeva M.A., Kondratenko E.C. About inulin structure Inulangrandis.// Chemistry of natural connections. -1982. -№2. –394-395pg.
Azimbaeva G.E., Kudaybergenova G.N., Izteleu B.M., Medeuova G.Dzh. The identification of inulin structure by physical-chemical method from Helianthus tuberosus. International Journal Of Applied And Fundamental Research. – 2014. – № 2 –
URL: www.science-sd.com/457-24650 (22.02.2026).











 PDF
PDF