About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
Summary
The phase composition, structures and properties of aluminum alloys were studied.
The optimal contents of alloying elements in alloys on the basis of Al-Cu-Mn-Zr system were determined . Presence of copper, magnesium and zirconium increases the temperature of liquids.
Results of calculation of temperature of liquids and solidus , which determine the modes of heat treatment, the temperature of melting and aluminum foundry are presented.
The ranges of concentrations and temperatures, at which the maximum number of dispersions can be reached, : Al20Cu2Mn3; Al3Zr; Al6Mn and the minimum number of phase Al2Cu, which correspond to the best heat-resistance of alloys, were determined.
Methods of thermo dynamic calculation, using the soft ware product Thermo – Calc and methods of SEM for quantitative assessment of phase composition and structure of aluminum alloys ,have been used in the work.
Keywords: alloy, concentration, property, diagram, liquids, solidus, heat resistance, wear resistance, electron microscopy.
Introduction. Known heat-resistant aluminum alloys , based on Al-Cu system, are such ones: casting alloys AM-5 and deformable ones of type 1201, Д16 ,AK4-1 (GOST 4784-94), their operational temperatures do not exceed 200-250ºС. [1]. Generation of new heat-resistant and wear-resistant aluminum-based alloys is an urgent problem.
Objective of the study is to examine phase equilibria in the system Al-Cu-Mn-Zr and determination of the optimal composition of the new heat-resistant aluminum alloy.
Task of the study : to find out the phase composition, structure and properties, based on knowledge of phase diagrams (PD) of the new aluminum alloy.
Methods of the study: in the work the methods of thermo dynamic calculation with the help of modern soft ware product Thermo-Calc, methods of SEM for quantitative assessment of phase composition and structure of aluminum alloys were used.
Results of the study: as an alternative to industrial alloys of type 1201 [1-5] in the studies a fundamentally new group of economically doped heat-resistant aluminum alloys (hereafter AЛТЭК) was suggested.
The main alloying components in the alloys of AЛТЭК group (as well as in industrial ones of type 1201) are copper, manganese and zirconium. Obviously, to make a well-founded choice of concentrations of alloying components and heat treatment modes analysis of at least (if not to take into account impurities and small additives) of the quaternary system Al-Cu-Mn- Zr is required.
Analysis of the compositions of alloys of type 1201 and AЛТЭК (Table 1) shows that the first one has a significantly higher concentration of copper, but lower concentrations of manganese and zirconium. This difference in the content of alloying components is what identifies the key difference between these groups, as discussed below.
Considering that the primary crystals of intermetallic compounds, generally undesirable, are formed at relatively small concentrations of transition metals, in the first stage (using database TTAL5) boundaries of liquidus for the thribble system Al-Cu-Mn were calculated. From Figure 1a it follows, that with the increase of copper content in the alloy the boundary of appearance of primary crystals of Mn-containing phases (Al20Cu2Mn3 and Al6Mn) shifts toward lower concentrations of manganese. This result is the first argument in favor of low coppery alloys AЛТЭК compared with alloys of type 1201, containing more than 6% of Cu. Calculation of boundaries of solidus also shows that with the increase of copper content the single-phase area(Al) narrows greatly in content of manganese: from 1,4% of Mn in the binary system to 0,2% of Mn at 5,7% of Cu (Figure 1 б ).
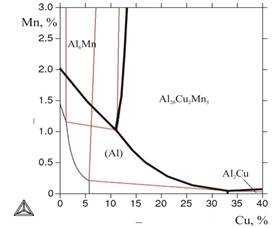
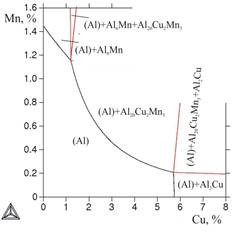
a b
Figure 1. Boundaries of surfaces of liquidus (marked with bold lines) and solidus in Al-Cu-Mn system: a)general view, b) solidus in the field of aluminum corner
Addition of zirconium into binary alloys of, as is known, leads to the formation of phase Al3Zr. Although in the literature there are no data on the diagram pattern Al-Cu-Mn-Zr, the distribution of phase areas in the aluminum corner of this quaternary system in the solid state can be predicted on the basis of available information. The four-quaternary alloys, depending on the ratios between the components, can have the following phase composition: (Al) + Al6Mn + Al3Zr (I); (Al) + Al6Mn + Al20Cu2Mn3 + Al3Zr (II): (Al) + Al20Cu2Mn3 + Al3Zr (III); (Al) + Al20Cu2Mn3 + Al2Cu + Al3Zr (IV). As shown in [2], area III is the optimal one.
Table 1 - Composition of some deformable alloys on the basis of Al-Cu-Mn-Zr system
|
Grade |
Cu, % |
Mn, % |
Zr, % |
Others |
|
D201 |
6,0–7,0 |
0,4–0,8 |
0,2 |
Ti |
|
12012 |
5,8–6,8 |
0,2–0,4 |
0,1–0,25 |
Ti, V |
|
АА 22193 |
5,8–6,8 |
0,2–0,4 |
0,1–0,25 |
Ti, V |
|
АLТEК4 |
1,2–2,4 |
1,2–2,2 |
0,15–0,6 |
Sc, V |
1OCT, 2 GOST 4784-97, 3 specification of Aluminum Association (USA), 4 RF Patent number 2252975 (publ. 27.05.2005, bulletin.№15
It is known that zirconium greatly increases the temperature of liquidus in binary alloys. Calculation shows, that presence of copper and magnesium little affects the extent of this increase, this is demonstrated by poly thermal sections ,presented in Figure 2, as well as the data presented in Table 2.
Temperatures of liquids (Tl) and solidus (TS) are one of the most important characteristics of any alloy. With the help of these temperatures the modes of thermal treatment, temperatures of melting and foundry of alloys are determined. Results of calculation of values of Tl and TS for certain alloys of system Al - Cu - Mn - Zr are presented in table 2, from which it follows that copper does not affect TL much , but significantly reduces TS. On the other hand, the addition of already 0,4% of Zr raises the liquids above 800° C.
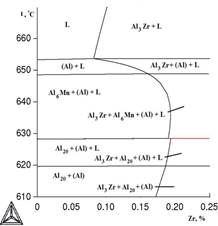
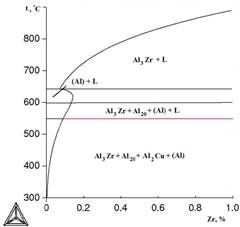
a b
Figure 2. Poly thermic sections of system Al-Cu-Mn-Zr at variable content of zirconium:
a) 2% of Cu and 1,5% of Mn; b) 6,5% of Cu and 0,5% of Mn
Table 2 – Parameters of crystallization of specific alloys of Al-Cu-Mn-Zr system
|
Cu, % |
tL, °C |
tS, °C |
Phases |
|
2 |
730 |
628 |
(Al) +Al20 +Al6 +Al3Zr |
|
5 |
731 |
576 |
(Al) +Al20 +Al3Zr |
Since the greatest effect from zirconium additive is connected with the formation of metastable phase Al3Zr with the crystal latitude L12 , the isothermal sections were calculated by excluding the stable phase (D023) from calculation . From section at 0,4% of Zr and 300 ° C (Figure 3) the sequence of changes of phase areas with increase of ratio Cu: Mn is seen.
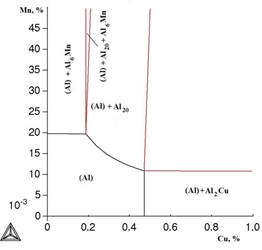
Figure 3. Isothermal section of the system Al-Cu-Mn-Zr at 0,4% of Zr and 300 °C:
calculation for metastable phase Al3Zr (L12)
Figure 4 presents poly thermal sections at variable contents of manganese and copper. It is evident that decrease of copper concentration from 2 to 1% reduces the probability of formation of phase Al2Cu.
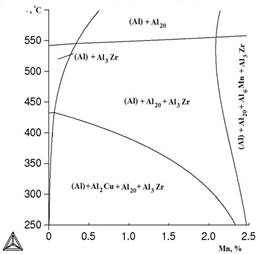
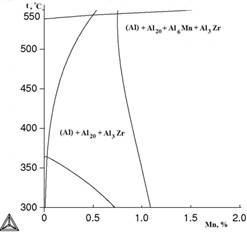
a b
Figure 4. Polythermal sections of system Al-Cu-Mn-Zr at 0,4% of Zr and variable content of manganese: a) 2% of Cu; b ) 1% of Cu: calculation for metastable phase Al3Zr (L12)
As is seen from Table 2, a small addition of copper has almost no influence on the nature of the alloy crystallization. In non-equilibrium conditions of crystallization the solubility of manganese in aluminum increases, and the formation of the ternary compound is suppressed. Therefore, in such alloys together with (Al) the phases Al2Cu and Al6Mn coexist. After the formation of primary crystals (Al), separation of phases Al2Cu and Al20Cu2Mn3 takes place according to the following reaction: L→ (Al) + Al2Cu + Al20Cu2Mn3 at 547 ° C. With further increase of copper concentration significant changes are not observed.
Quantitative information about the phase composition of characteristic alloys at 350 ° C and 540 ° C is presented in Table 3.
Table 3 - Phase composition of alloy at Al - Cu - Mn - Zr at 350 ° C and 540 ° C, calculated according to Thermo-Calc program
|
Phase |
% mass |
% volume |
Content of components, % |
|||
|
Al |
Cu |
Mn |
Zr |
|||
|
t = 350 °C |
||||||
|
(Al) |
85,74 |
88,72 |
99,1 |
0,86 |
0,02 |
0,008 |
|
Al20 |
11,53 |
8,95 |
64,89 |
15,28 |
19,82 |
0 |
|
Al2Сu |
2,70 |
2,3 |
46,36 |
53,63 |
0 |
0 |
|
Al3Zr |
0,04 |
0,02 |
47,01 |
0 |
0 |
52,98 |
|
Alloy |
100 |
100 |
Base alloy |
|
|
|
|
t = 540 °C |
||||||
|
(Al) |
88,11 |
90,81 |
96,78 |
2,85 |
0,26 |
0,09 |
|
Al20 |
11,85 |
9,16 |
64,89 |
15,28 |
19,82 |
0 |
|
Al3Zr |
0,04 |
0,03 |
47,01 |
0 |
0 |
52,98 |
|
Alloy |
100 |
100 |
Base alloy |
|
|
|
Conclusion.
1. Using Thermo-Calc program, for the first time a sequence of poly thermal and isothermal sections of phase diagram of system Al - Cu - Mn - Zr in the range of compositions of alloys : 2 ¸ 5% of Cu, 1¸ 3% of Mn, 0,4 ¸ 0,6% of Zr ,the rest Al. was formed . Analysis of phase composition of the alloys was carried out, the optimum contents of alloying elements were determined. Optimal compositions of new industrial alloys of AЛТЭК type were suggested.
2. Areas of concentrations and temperatures, at which the maximum number of dispersions Al20Cu2Mn3, Al3Zr, Al6Mn can be achieved and minimum quantity of phase Al2Cu, which corresponds to the best heat-resistance of alloys, are determined.
3. It is shown, that zirconium violently increases the temperature of liquids of the alloys, regardless of the concentrations of copper and manganese. It is necessary to melt alloys of AЛТЭК type at much higher temperatures, compared with commercial alloys.
4. The optimal compositions of new aluminum alloys, intended for production of shaped castings and deformed semi-finished products on the existing equipment are presented. The main advantages of the new alloys over the branded ones in combination of operational and technological parameters are presented.
2. Belov N.A. Phase composition of aluminum alloys: Scientific publication. - M .: Publishing House MIS&A, 2009. - p. 392
3. Belov N.A., Alabin A.N. Promising aluminum alloys with additions of zirconium and scandium. Non-ferrous metals, 2007, №2, p.99-106.
4. Belov N.A., Smagulov D.U., Nurumgaliyev A.K., Toleuova A.R. Promising aluminum alloys with addition of zirconium and scandium. Proceedings of the IIIrd International Kazakhstan metallurgical conference "50 years of Kazakhstan magnitka." Section "Innovative Technologies", Temirtau, KarSIU , 2010, pp. 53-56
5. Amenova A.A.,Toleuova A.R., Belov N.A., Smagulov D.U. Calculation of isothermal sections of the phase diagram of the system Al-Cu-Mn-Zr -Fe - Si. " RK NAS news." Almaty. №6 (434) 2011 pp.82-85.Section: Methods and technology.
Islamkulov K.M., Myrkhalykov Zh.U., Amenovа А.А., Smagulov D.U. DEVELOPMENT OF HEAT-RESISTANT AND WEAR-RESISTANT ALUMINIUM ALLOYS. International Journal Of Applied And Fundamental Research. – 2016. – № 1 –
URL: www.science-sd.com/463-24974 (31.01.2026).











 PDF
PDF