About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Engineering
The direct action methods by concentrated streams of energy on details working surfaces derive more and more wide applying now, because the traditional technological methods stipulating predominantly the improvement of bulk properties materials (thermal, thermo and chemical and other kinds of processing) have already exhausted their possibilities.
One of such methods is the process of details surface strengthening, predominantly from aluminum alloys, based on microarc oxidation (MAO). The MAO represents rather new and perspective method of surface hardening which has arisen in the beginning of the seventies due to discovery of microarc discharges phenomenon in an electrolyte developed in the Institute of Inorganic Chemistry of Siberian Department of Russian Academy of Sciences (author Markov G.A.) [1-6]. This method allows forming on the surface the fundamentally new high-quality coatings with high wear resistance and good adhesion to substratum. Due to their unique properties, a combination of high wear resistance, corrosion resistance, and also thermo- and erosion resistance, all the more wide areas of different branches of machine industry find practical use of new coatings. Other positive distinctive features of MAO-process are its environmental friendliness and also absence of necessity of preliminary surface preparation in the beginning of technological chain and applying of refrigerating machinery for deriving concerning thick coatings.
It should be said that in the technical literature for a long time already is debated the question of the method’s name. It is called either microarc oxidation [1,5,6] or microplasma method [7] or plasma electrolytic oxidation [8,9]. Of course, if we will consider the method in terms of its differences from conventional anodizing, then the presence of discharges on the surface during processing should be considered as the main hallmark of this method. From these positions the name of the method as plasma electrolytic oxidation is quite justified. On the other hand, the action of the spark discharges on the surface of material distinguishes from the action of microarc discharges and even more from the action of arc discharges on discharges energy and on quality of the formed coatings. In this regard, the coatings formed under conditions of microarc discharges are the best in terms of quality (high values of physical and mechanical characteristics, hardness, wear resistance, etc.), and therefore the method’s name as microarc oxidation is the most appropriate.
The possibilities of treatment by microarc oxidation method can be described by applied current modes, most preferable of which ones are represented on Fig. 1.
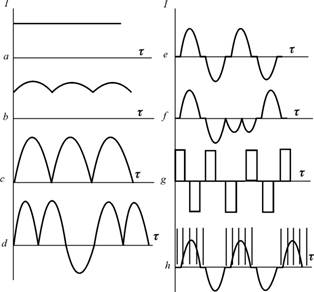
Figure 1. Characteristic curves for currents modes of aluminum alloys at the microarc treatment: a – direct current; b,c – pulse current with different ripple factor; d – anode pulsating with reverse impulses; e – anodic and cathode current of industrial frequency; f – combined anodic and cathode current with cathodizing; g – anodic and cathode pulses; h – anodic and cathode current with submission of high frequency pulses.
First three (a,b,c) modes are characteristic for anodic MAO with different ripple factor (from 3 up to 100 %) [4,6]. A mode d - anodic pulsating with submission through each 5-50 positive impulses one negative [5]. Anodic-cathode modes (e,f), including usual anodic-cathode MAO (ACMAO) and ACMAO with cathodizing (C), realized on the power supply with an industrial frequency f =50 Hz [3,10,11]. The last group of modes (g,h) – different impulse submissions of a current with frequency exceeding industrial, as a rule, up to 1 - 2 кHz. As a whole, the method of microarc oxidation is a complex process which characteristics are depended both from external factors (component composition, concentration, рН and temperature of electrolyte; mode of MAO: polarity, frequency, amplitude and form of voltage and current impulses, their ratio; treatment time etc.), and from internal factors (composition of alloy, its heat treatment, structure, porosity of oxidizing material etc.). These factors define the thickness of coatings, their composition, structure, density and porosity, micro hardness, bond strength with a basis, wear- and corrosion resistance, electro- and thermal conduction, breakdown voltage and other properties [12,13].
The goal of this paper is to show the possibilities of some modes of microarc treatment to enhance the main properties of details work surfaces especially their wear resistance in different operating conditions.
- Methods and materials for investigations
Because MAO-coatings have rather high mechanical characteristics (microhardness, elastic modulus etc.), for fast receiving of an estimation of their wear resistance the method of trials on abrasive outwearing is most acceptable [14]. The estimation of relative wear resistance was carried out on the installation [15] designed by author on the friction schema “pin-on-disk” at the outwearing of coating samples on fastened abrasive particles (abrasive wheel from green silicon carbide, with graininess <0.070 mm). The samples (pin) were made from aluminum alloy 2024 (Д16) or steel 40. The friction was carried out all time on fresh abrasive surface, due to radial displacement of sample on rotating abrasive wheel with velocity 0.3 mm/rev (path of friction was Archimedes spiral). Simultaneously, there was carried out the cleaning of friction surface with help of diamond abrasive wheel. The products of wear were deleted from the surface by submission of water in a zone of friction. The specific load on the sample was changed from 0.25 up to 1.25 MPa. Each test was conducted during 30 sec that corresponded 22 m of friction path. The velocity of sliding within trials was changed from 0.6 up to 1.0 m/s. The wear was evaluated on a change of mass (on analytical balance W21 with exactitude ±0.0001 g) and linear by means of indicator 1IGM (1ИГМ) with an exactitude ±0.001 mm. The applying of two methods wear estimation has allowed us to control gross errors in measurements and to increase a level of reliability of the obtained data.
- MAO-coatings wear resistance
A plenty of materials and coatings that are applied for increasing wear resistance of friction knots is known now. Among them it is possible to mark the composites on the basis of tungsten carbides with copper-nickel bunch, different surface hardening materials, such as PG-KhN80Sr4 (ПГ-ХН80Ср4), VSNGN (ВСНГН) etc., siliconized graphite of the marks SG-P, SG-M, SG-T (СГ-П, СГ-М, СГ-Т), mineral ceramic TSM -332 (ЦМ-332) on the basis of Al2O3, cemented-carbide compositions like VK-6, VK-8, T15K6 (ВК-6, ВК-8, Т15К6) [16,17] and others. The wear resistance of MAO-coatings was evaluated compared to following materials: composite on the basis of tungsten carbides WC-Cu-Ni, siliconized graphite SG-P, and also cast iron SCH18 (СЧ18), steel 45, hardened up to HRC44 and steel 3 as the etalon.
The results of these tests are shown on Fig. 2. One can see a relatively high wear resistance of MAO-coatings in comparing to tested materials. Moreover, characteristically that the linear wear grows proportionally with increase of specific load on contact down to Psp=1.5 MPa, while for cast iron, steel 45 and steel 3 at the loading over 1.00 MPa already the more wear is watched.
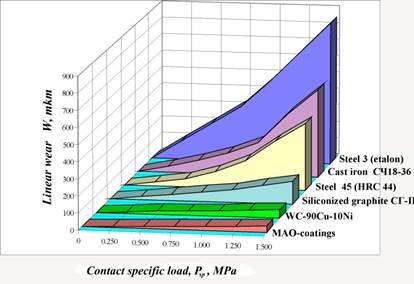
Figure 2. Comparable wear resistance of tested materials and coatings
Great interest represents also comparison of tests outcomes wear resistance of MAO-coatings with data of wear resistance investigations of materials carried out by other investigators [17,18]. Such comparison allows us clearly to evaluate a relative level of ceramic coatings wear resistance to contrast of wide range of materials. For realization of this, the data points of MAO-coatings relative wear resistance were entered onto a graph of relative wear resistance ε from the ratio of hardness of abrasive particles and tested materials Ha/Hm (see Fig.3). Besides, for expansion of comparison spectrum on the same graph were put the data of relative wear resistance for different hardening coatings, surface hardening welding and modified layers received by modern methods of materials processing such as PVD-technologies [19], laser hardening [17] etc. with respect to the wear of the selected etalon steel 3.
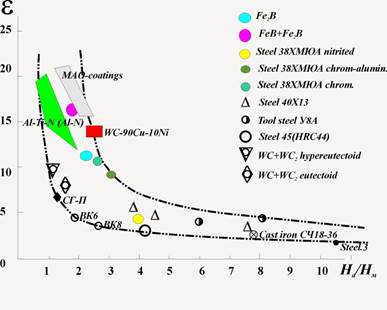
Figure 3. Dependence of relative wear resistance e of different materials, hardening layers and coatings from hardness ratio of abrasive and materials Ha/Hm.
From the Fig.3 one can see that the MAO-coatings have a very high wear resistance comparable to wear resistance of materials and coatings traditionally used against an abrasive wear: WC-Cu-Ni, boron layers (FeB, Fe2B), ionic-vacuum coatings.
In [18] is specified that considerable increase of materials wear resistance is reached by introduction in it the high-tensile inserts. The big difference in properties α- and γ-Al2O3 allows considering as such high-tensile inserts in MAO-coating the phase α-Al2O3, due to which its high wear resistance is ensured. Thus, the complex of MAO-coating phases of alumina and other components represent some disperse system composed of a disperse phase (α-Al2O3), in a certain way distributed in dispersion (matrix) medium, or, the matter of fact – the composite material [20].
- Wear resistance of MAO-coatings formed on ferrous metals
Obviously, the primary use of MAO method relates to different valve metals and their alloys, namely aluminum, titanium, manganese, zirconium, niobium etc., but we know that the most details of machines are made from steels which do not are the valve metals. Therefore, it is appear some difficulties at the formation MAO-coating on such metals. In this case for realization the MAO-process on ferrous metals there are needed first to form on the surface a pre-film from any organic or inorganic material with dielectric properties and then use usual electrolyte for MAO-treatment. But, because the iron oxides have relatively weak mechanical properties as compared to aluminum oxides, such method does not provide to obtain wear resistant coatings on ferrous metals. On the other hand, the formation of wear resistant coatings on ferrous metals can be received by way of pre-forming on their surfaces the coating from aluminum alloy with thickness up to 250-300 μm and its further microarc processing in electrolyte [21].
The essential here is that the MAO-treatment should be carried out to a depth of 2/3 of the aluminum layer thickness and not on whole thickness of this aluminum layer. Only in this case we can obtain high quality coatings on steels (see Table).
Table
Formation conditions and properties of MAO-coatings on ferrous metals
|
Parameters |
Examples of Implementation |
||||
|
|
1 |
2 |
3 |
4 |
5 |
|
Aluminum containing layer: Layer thickness, μм |
2024 300 |
2024 300 |
2024 300 |
2024 300 |
2024 300 |
|
Microarc oxidation in electrolyte: 1 g/l КОН+ 8 g/l Na2SiO3 Final anodic voltage, V |
700 |
650 |
650 |
650 |
650 |
|
Final cathode voltage, V |
150 |
130 |
130 |
130 |
130 |
|
Current density, А/dm2: Anodic |
10 |
10 |
10 |
10 |
10 |
|
Cathode |
11 |
11 |
11 |
10 |
10 |
|
MAO-coating thickness, μм |
300 Yellow spots on the surface |
210 |
190 |
200 |
220 |
|
Bond strength, MPa |
22 |
45 |
28 |
25 |
34 |
|
Relative wear resistance |
7,9 |
16,2 |
9,3 |
8,2 |
11,3 |
Thus, in cases when it is necessary, the use of hybrid technology allows forming on the work surfaces of parts made from structural steels or other ferrous metals, wear resistant ceramic coatings, which are comparable in their strength properties with MAO coatings formed on aluminum alloys and enhance their wear resistance in 2-2.5 times.
- Formation of microarc coatings in slurry electrolytes
The technology of coatings formation in electrolytes-suspensions represents one of perspective directions of MAO-technique because it allows obtaining the composite materials on treated surfaces of working pieces. The combinations of powder materials entered into electrolyte are contained in the compositions of these MAO-coatings [5,22]. Today this technology is represented by a separate group of electrolytes which have the most complex composition. The MAO-coatings formed in such slurry electrolytes contain a significant share of powder material entered into electrolyte as particles of disperse phase (DP). Different nonmetallic combinations: oxides, carbides, borides, nitrides of metals are used as particles of disperse phase. The sizes of entered particles vary in rather broad limits: from nanometers (ultra dispersed components) to 0.1–100 mm. The concentration of particles in electrolyte can be from 5 to 100 g/l.
The slurry electrolytes were prepared by dissolution of necessary amount alkali (potassium hydroxide) in distilled water and introduction in them the calculated amounts of dissoluble salts and disperse phase (oxides, carbides, nitrides, borides of metals) which previously were mixed by wet method (in a ball mill) with the calculated amount of ultra dispersed powder (for example, aerosil-amino).
The electrophoresis [23] in its pure kind, as a rule, does not take place in anodic–cathode microarc treatment mode because of alternating electrodes polarization and influence of other numerous factors also. But the particles of disperse phase which found themselves in a zone of microarc discharge are being involved in a volume treated by microarc and can be built-in by one or another way into a structure of formed coating. The properties of such composite material essentially will depend from the status of powder material entered into electrolyte which could be found in a coating: either in an unchanged status or in combinations with other elements, or built-in in a crystal lattice of oxide. First of all it will depend on a type of combinations and their kind of bond between elements. Unlike typical standard electrolyte, the MAO-coating formation process in such slurry electrolytes has some other mechanism. The main difference of this mechanism is the following: due to the presence of weighed particles in an electrolyte, all processes of surface film formation intensify sharply [22]. The presence of solid particles in electrolyte provide modification of formed coating in such a manner that powder materials entered into electrolyte are being detected in a composition of ceramic film surface, not only visually, but also by X-ray diffraction method.
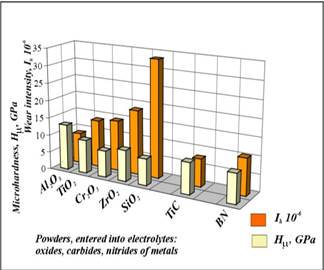
Figure 4. Comparative data on microhardness and intensity of abrasive wear of MAO-coatings formed in slurry electrolytes with added powders of oxides, carbides, borides and nitrides of metals.
Some results on microhardness study and tests on abrasive wear resistance of MAO-coatings formed in slurry electrolytes are shown on Fig.4. Analyzing the obtained data it is possible to mark that the coatings formed in slurry electrolytes have a wear resistance practically the same as coatings formed in ‘‘pure’’ standard electrolytes. But the duration of formation process is decreased significantly (in 2–3 times). So, for example, the duration of coating formation with thickness more than 150 μm in some conditions lasts 30–40 min, while for usual electrolytes this process continues 120–150 min.
Conclusions
For MAO-process are characteristic the high temperatures in discharge channels and, as consequent, the formation of high-temperature phases in coatings (for aluminum alloys it is a hard corundum - α-Al2O3); the destruction of water with formation of atomic and ionized Oxygen; local magnification of electrolyte concentration and particular plasma chemical reactions in a zone of discharge; local sequential transformation in discharge of oxides formed by electrochemical way.
The structure and composition of oxide layers, apart from the nature of treated metal and other little significant internal factors, is defined by the environmental conditions of their formation and especially by composition of electrolyte components which can enter into coating structure, by time and technological parameters of processing mode defining thermal, temporary, and other characteristics of microdischarges.
The introduction into electrolyte a powders of disperse phase, besides improving coatings properties, allows to intensify all stages of coatings formation process considerably, diminishing duration of exposition in 2–3 times.
2. Markov G.A., Shulepko E.K., Terleeva O.P. Method of coatings deposition on metals and alloys. Publish. Bull. Inv. 13, 1989. Copyright Certificate 1200591 C 25D 11/02 [in Russian].
3. Markov G.A., Gizatullin B.S., Rychazhkova I.B. Method of electrolytic deposition of silicate coatings. Publish. Bull. Inv. 17, 1982. Copyright Certificate 926083 C 25 D 11/02 [in Russian].
4. Markov G.A., Shulepko E.K., Zhukov M.F., Petshevitsky B.I. Method of metalsand alloys oxidation. Publish. Bull. Inv. 17, 1982. Copyright Certificate 926084 C 25 D 11/02 [in Russian].
5. Markov G.A., Belevantsev V.I., Terleeva O.P., et all. Microarc oxidation. Vestn. MSTU, Ser.Machinostr. 1, 1992, 34-56 (ISSN 0236-3941) [in Russian].
6. Markov G.A., Terleeva O.P., Shulepko E.K. Microarc and arc methods for deposition of protecting coatings. I.M.Gubkin MINKH and GP, Collection of Works, Moscow, vol.185, 1985, 54-66 [in Russian].
7. Markov G.A., Belevantsev V.I., Slonova A.I., Terleeva O.P. Stagety in anodic-cathode microplasma processing. Electrochemie. 1989, Vol. 25, № 11, 1473-1479 [in Russian].
8. Yerokhin A.L., Nie X. and Leyland A. et al., Plasma electrolysis for surface engineering, Surf. Coat. Technol. 122 (1999) (2–3), pp. 73–93.
9. Yerokhin A.L., Snizhko L.O., Gurevina N.L., Leyland A., Pilkington A. and Matthews A. Discharge characterization in plasma electrolytic oxidation of aluminium, J.Phys.D: Appl.Phys. 36 (2003), pp. 2110-2120.
9. Mc Neil W, Wick R. Effect of various polyvalent metal anion addition to an alkaline magnesium anodizing bath. J. Electrochem Soc. 1957, V. 104, № 6, 356-359.
10. Mc Neil W., Gruss L.L. Anodic film growth by anion deposition in aluminate, tungstate and phosphate solutions. J. Electrochem. Soc. 1963, V. 110, № 8, 853-855.
11. Mc Neil W., Gruss L.L., Husted D.G. The anodic synthesis of CdS films. J. Electrochem. Soc. 1965, V. 112, № 7, 713-715.
12. Timoshenko A.V., Opara B.K. Kovalev A.F. Microarc oxidation of alloy D16 on alternative current in alkali electrolyte. Protection of metals. 1991, Vol.27, №3,.417-424 [in Russian].
13. Aluminum alloys. Structure and properties of half-finished products from aluminum alloys. Handbook. Metallurgy: Moscow, 1974. p. 432. [in Russian].
14. Khrutshev M.M., Babichev M.A. Abrasive outwearing. Nauka: Moscow, 1970, p.252 [in Russian].
15. Malyshev V.N., Golub M.V., Kharlamenko V.I. Investigation of composite materials relative wear resistance. VNIIOENG. Machines and oil equipment, 1983, № 4, 6-8 [in Russian].
16. Katsheev V.N. Processes in a zone of metals friction contact. Mashinostroenie: Moscow, 1978, p.213 [in Russian].
17. Vinorgadov Yu. M. Wear resistant materials in chemical machine industry. Handbook. Mashinostroenie: Leningrad, 1977, p. 256 [in Russian].
18. Khebda M., Chichinadze A.V., et all. Handbook on tribotechnic. Theoretical basis. Mashinostroenie: Moscow, 1989, Vol.1, p.400 [in Russian].
19. Semenov A.P., Kovsh I.B., Petrova I.M. et all. Methods and means of details machines surfaces hardening by concentrated flows of energies. Nauka: Moscow, 1992, p. 404 [in Russian].
20. Fedorchenko I.M., Pugina L.I. Composite heated antifriction materials. Naukova dumka: Kiev, 1980, p.404 [in Russian].
21. Malyshev V.N. Formation of wear resistant coatings on ferrous metals and alloys by micro-arc oxidation technology // Hardening technology and coatings, 2006, №12, 32-38 [in Russian].
22. Malyshev V.N., ZorinK.M. Features of microarc oxidation coatings formation technology in slurry electrolytes. Applied Surface Science, 2007 (254), 1511-1516
23. Dukhin S.S., Deryagin B.V. Electrophoresis. Nauka: Moscow. 1976, p.332 [in Russian].
Malyshev V.N. ENHANCE OF WORK SURFACES WEAR RESISTANCE BY MICROARC OXIDATION. International Journal Of Applied And Fundamental Research. – 2016. – № 3 –
URL: www.science-sd.com/465-25003 (11.02.2026).











 PDF
PDF