About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Biological sciences
Neuro-endocrinal reactions form a foundation of adaptive response among towards environmental factors among animals, therefore, endocrinal research is applied in studying aspects of reproduction, influence of stress and different ecological factors for many groups of animals. One of key indicators of animals’ health is level of gluco-coticoids. Estimation of adrenal cortex activity is traditionally held on blood samples [6;12]. However, in many cases collection of blood sample is a stress itself, and thus can have an effect upon result. Definition of hormones in blood serum and plasma can be replaced with methods, based upon estimating hormone levels in fecal mass, saliva, urine, and fur. During the recent two decades methods of analyzing fecal steroids (estrogen, androgen, gestagen, and gluco-corticoid metabolites) have become widely-used in research of mammals [1;2;3;11], birds [5], reptiles [4], amphibias [13], and fish [16]. Shares and structure of gluco-corticoid metabolites that are discharged with urine and fecal mass, are different even among close types of mammals, therefore, it is necessary to validate methods of estimating gluco-corticoids for each kind separately [15].
Nowadays many types of rare and extincting kinds of animals are preserved I zoos and on controlled territories in wild conditions. In all these cases control over condition of anmals is required, thus, defining concentration of gluco-corticoids can be used as index of animals’ welfare.
European mink (Mustela lutreola) is one of the rarest kinds of mammals at European continent [14]. The kind is preserved by keeping it in captivity, but reproduction of it faces a number of problems, among them is low sexual activity of male animals, reduced adaption of animals in case of their return to wilderness [9]. In forming foundation groups for natural populations emerges a problem selecting animals that are able to adapt to new conditions from cage population, and defining basic concentration level of gluco-corticoids as well as studying their cyclic nature allows us to analyze individual features of each animal, initiates their “stress resistance”. Collecting blood samples from European mink is related to great difficulties due to heavy stress for each animal, whilst defining gluco-corticoid level from excrement samples is a simple procedure, has no influence upon an animal, and allows for systematic regular examinations. Objective of this research is to define basal concentrations of cortisol and testosterone in fecal mass of cage European mink and study oscillations in this hormones’ level among different sexual and age groups and separate animals
Methodic
The mink were kept separately by one specimen in standard cages with cell 2x4cm, size 20x45x90cm. Every cage was connected to wooden nest box (28x20x40cm), and placed in shed. With a special hatch exiting nest box and cage walk could be terminated without bothering animal. The animals were fed once a day in the first half of it. Forage consisted of meat products, fish, and rodents. Fresh water was constantly available in cages. Totally 22 European minks of different age (from 10 moths to 8 years old) participated in experiments (Table)
Table
Age and sex of European minks participating in experiments and amount of collected experiments
|
№№
|
Sex |
Number of animal
|
Duration collecting of excrements, hour |
Number number |
Age of animal years (months) |
|
1 |
male |
1153 |
72 |
13 |
5 years |
|
2 |
male |
43 |
70 |
3 |
3 years |
|
3. |
male |
45 |
84 |
8 |
10 mon. |
|
4 |
male |
22 |
8 |
3 |
3 years |
|
5 |
male |
30 |
8 |
3 |
2 years |
|
6 |
male |
35 |
4 |
2 |
10 mon. |
|
7 |
male |
40 |
4 |
2 |
10 mon. |
|
8 |
male |
1 |
30 |
5 |
4 years |
|
9 |
female |
41 |
72 |
3 |
3 years |
|
10 |
female |
42 |
72 |
5 |
3 years |
|
11 |
female |
1242 |
72 |
16 |
8 years |
|
12 |
female |
44 |
72 |
15 |
10 mon. |
|
13 |
female |
1372 |
72 |
14 |
5 years |
|
14 |
female |
14 |
30 |
5 |
3 years |
|
15 |
female |
1336 |
30 |
5 |
6 years |
|
16 |
female |
18 |
28 |
6 |
3 years |
|
17 |
female |
25 |
16 |
4 |
3 years |
|
18 |
female |
12 |
20 |
5 |
3 years |
|
19 |
female |
15 |
20 |
5 |
3 years |
|
20 |
female |
19 |
16 |
4 |
3 years |
|
21 |
female |
33 |
24 |
3 |
2 years |
|
22 |
female |
38 |
15 |
3 |
11 mon. |
It attributed the young animals, whose age is 11 months and smaller, adults believed to be animals of 12 months and older. Collection of feces was performed in 2014 before the start of the breeding in the period from 15 March to 2 April. Before sampling old feces were removed from the cells. During the daylight hours the cells of animals examined each hour, and collected feces. Collection of feces in individual animal was carried out for 2-3 days. At night, we managed to collect only single feces samples. The collected feces were frozen and stored at -20 °. In total were collected 132 fecal samples. Collected feces after complete drying, were extracted into ethyl alcohol 40 mg / ml. After settling week with periodic vortexing samples were centrifuged 10 minutes at 2000 rev / min, and selected 50 to 300 .mu.l further define respectively testosterone and cortisol. After drying in batches of a test tube was added 100 ul of phosphate buffer, heated for 5 min at 400C and centrifuged for 5 min at 2000 rev / min. Further determination of hormone concentration was carried out according to the instructions kits for enzyme immunoassay Testosterone EIA-Best and Cortisol-EIA-Best (Vector-Best, Novosibirsk). To determine optical density spectrophotometer Uniplan was used (AIFR-01). The data were converted to 1g of dry feces.
From the group of animals from which the feces were collected, 6 males and 9 females were involved in reproduction in 2012-2015., making it possible to determine the sexual activity of these animals. The willingness of females to mate was determined by vaginal smears. The significance of difference between indexes was determined by the U criterion by Mann-Whitney. Differences were considered statistically significant at p <0,05.
Results and discussion
Cortisol
Determination of cortisol levels in the feces of the European mink has revealed significant variations in its concentration during the day. Fluctuations in the value range of cortisol in young males ranged from 2.1 to 41.5 g / g, Adults: 0,4-22,0 ug / g. Cortisol values close to the maximum: 18-22 ug / g were observed in males at different times of the day - from 9:00 to 19:00. The highest amount of cortisol which is equal to 41.5 mg / g awarded once at 12:00 in the male number 45, the lowest concentration of hydrocortisone 2.1 mcg / g in the same male found in the feces collected in the night 3:40 (Fig. 1). During the 2014 breeding season in 10-11months old and in 2015 at the age of about 2 years of repeated attempts to plant female to male it ended in failure. The male was extremely aggressive with respect to different females. It should be noted, that some of the males reached the 10 month during encounters, with the females was also observed aggressive behavior, but at the age of 2-3 years, aggression toward females tend to disappear.
The scope of cortisol values fluctuations in females: 0,1-44,7 ug / g, which is different from the range of fluctuations in males. The difference between the maximum and minimum values of cortisol in males and females are statistically significant (p <0.05).
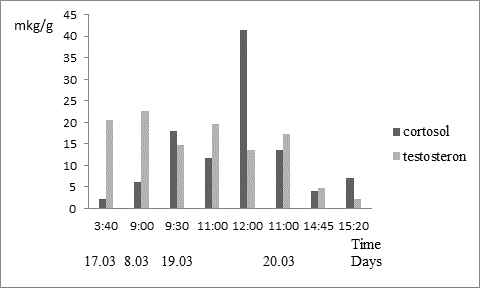
Fig.1. Dynamics excretion of cortisol and testosterone the male European mink No. 45 for four days: 17.03–20.03.2014; magnitude of testosterone decreased in 4 times.
A female №1372 two cortisol peak recorded in three days: in excrement samples collected on 03/21 at 14:30 pm. and on 03/22 at 12:00 pm. During the same period the value of cortisol time on the following day was 2.5-3 times less (Fig.2). The female №1242 also registered two peaks of cortisol on 03/21.03 at 11:30 pm and 03/22.03 at 12:00 pm. The next day in the period from 11:00 to 14:00 h. cortisol value was 4 times less. The maximum and close to the maximum value of cortisol 26.0 - 44.7 ug / g were recorded in 5 adult females in the samples obtained in the morning from 8:00 to 12:00 h. For two adult females were obtained data on the content of cortisol excretion at night: at 3:40 am - 22 g / g and 4:00 am - 2.1 mg / g, a young female number 38 cortisol levels in the feces collected at 4:00 hour. It was 0.2 mg / g. As a rule, in the night hours’ mink were inactive, which is likely due to cell content and the fact that the feeding of animals was timed to the day-time.
Testosterone
Fluctuations in testosterone values range from ten months amounted to 17,0-91,0 males, males of 2 years and older: 16,0-176,7 ug / g, female: 6,1-185,4 ug / g (Fig. 1-2). There was no confinement of the maximum and minimum values of testosterone to a specific time of day. At different days at the same time of day values of testosterone can vary 5-6. The difference between the maximum and minimum values for males and females (p <0.05) are valid.
Considerable daily fluctuations in cortisol concentrations in the feces in the European mink were detected. Cortisol is the main corticosteroid, which responds to the stress factor in a mammal. Dynamics of cortisol in repose shows the animal endocrine profile, creating the basis for further
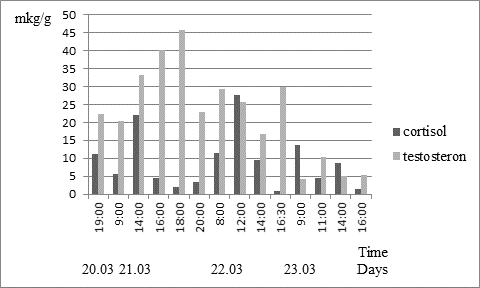
Fig.2. Dynamics excretion of cortisol and testosterone the female European mink No. 1372 for 20.03–23.03.2014; magnitude of testosterone decreased in 4 times
monitoring of possible animal as the experimental conditions, and in the wild. For many species of animals and humans set about 4-day biorhythm dynamics levels of glucocorticoid hormones and testosterone, indicating the universality of 4-day biorhythm levels of glucocorticoid hormones in mammals [8].
Fluctuations in the level of fecal glucocorticoid metabolite cannot show a clear rhythm as in the measurement of free glucocorticoids in the blood, because corticosteroids concentration determined by measuring fecal metabolites, depends on the quality and quantity of food eaten by the animals and the time of defecation, i.e. glucocorticoids metabolites in feces is integrated over time. Nevertheless, the level of fecal metabolites glucocorticoids correlates well with the original free in plasma glucocorticoids [7; 12]. In cell fur-bearing animals as well as wild animals breeding, molting, metabolism is dependent on external factors, the length of daylight and temperature conditions. We observed sex differences in confinement maximal cortisol values to the different time of day, it can be explained by differences in daily activity in males and females.
Under natural conditions, European female mink active both during the day and at night, while 82.5% of the activity accounts for male night-time, although there is a great individual variability in activity [10]. In studying the effects of various factors, assessment of the functional state of the endocrine system of the European mink and their individual differences based on the measurement of fecal cortisol is necessary to consider not individual values ??of fecal cortisol and interval indicators, taking into account jet lag, which, of course, complicates the process of investigation, otherwise one - two randomly taken sample may give a distorted picture of the state of the test animal and the effect of the studied factors.
Using the results obtained, it can be assumed that the values of the concentration of cortisol in males and particularly the maximum values can be used as an indicator of the functioning of the hypothalamic-pituitary-testicular axis. Therefore, it is likely that in the practice of breeding by establishing cortisol concentrations in the feces can diagnose the fertility of males, although this issue requires further study.
2. E.L. Zavialov, L.A. Gerlinskaya, V.I. Evsikov Evaluating stress level of red-backed mouse Clethrionomys glareolus (Rodentidae, Rodentia) according to corticosteron in fecal mass // Zoological magazine, 2003, v. 82, № 4, p. 508–513.
3. E.V. Pavlova, S.V. Naydenko Special features of adreno-cortical system basal activity and its relation to behavior of far-East forest cat. (Prionailurus bengalensis euptilura) // Zoological magazine, 2012, v.91, № 10, p. 1261 – 1272.
4. Atkins N., Jones S.M., Edwards A. Fecal testosterone concentrations may not be useful for monitoring reproductive status in male blue-tongued lizards (Tiliqua nigrolutea: Scincidae) // J. Herpetology. 2002. V. 36. P. 106–109.
5. Baltic M., Jenni-Eiermann S., Arlettaz R., Palme R. A noninvasive technique to evaluate humangenerated stress in the black grouse // Annals of the New York Academy of Sci. 2005. V. 1046. P. 81–95.
6. Cook N. J. Review: Minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals // Cаnadian J. Animal Sci.2012.V.92. P. 227–259 (doi:10.4141/CJAS2012-045).
7. Eriksson H., Gustafsson J.A. Steroids in germfree and conventional rats. Distribution and excretion of labelled pregnenolone and corticosterone in male and female rats // Eur. J. Biochem. 1970. V. 15. P. 132–139.
8. Jozsa R., Olah A., Cornélissen G., Csernus V., Otsuka K., Zeman M., Nagy G., Kaszaki J., Stebelova K., Csokas N., Pan W., Herold M., Bakken E. E., Halberg F. Circadian and extracircadian exploration during daytime hours of circulating corticosterone and other endocrine chronomes // Biomed. Pharmacother. 2005. V. 59. № 1. P. 109–116.
9. Kneidinger N., Schwarzenberger F., Nagl A., Kiik K., Ashford K., Maran T. The use of non-invasive monitoring methods in conservation breeding of the endangered European mink (Mustela lutreola)//Veterinary Medicine Austria Non-invasive Monitoring of Hormones” (3rd annual ISWE meeting). Austria. 2012. P. 54.
10. Miñano S.P., Ruiz-Olmo J., Noguera J. G. Activity patterns of European mink in Iberian Peninsula//Euro-American Congress, Santiago de Compostela, Spain, July 19–24 1998. P. 265.
11. Schwarzenberger F. The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species//International Zoo Yearbook. 2007. V. 41. P. 52–74.
12. Sheriff M. J., Krebs C. J. Boonstra R. Assessing stress in animal populations: do fecal and plasma glucocorticoids tell the same story? // Gen Comp. Endocrinol. 2010. V. 166. P. 614–619.
13. Szymanski D. C., Gist D. H., Roth T. L. Anuran gender identification by fecal steroid analysis // Zoo Biology. 2006. V. 25. P. 35–46.
14. The IUCN Red List of Threatened Species. Version 2014. 3. www.iucnredlist.org>
15. Touma C., Sachser N., Möstl E., Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice//General Comp. Endocrinol. 2003. V. 130. P. 267–278.
16. Wysocki L.E., Dittami J.P., Ladich F. Ship noise and cortisol secretion in European freshwater fishes//Biol. Conserv. 2006. V. 128. P. 501–508.
Kiseleva N.V., Kondratyuk E.Y. ASSESSMENT OF VARIABILITY OF CORTICOSTEROIDS OF THE EUROPEAN MINK (MUSTELA LUTREOLA) USING NON-INVASIVE METHODS. International Journal Of Applied And Fundamental Research. – 2016. – № 4 –
URL: www.science-sd.com/466-25078 (11.02.2026).









 PDF
PDF