About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Medical sciences
INTRODUCTION
Research of new ordinary, rational approaches to prevention and treatment of infectious pathologies using advanced methods of chemotherapy, the creation of new and effective microbicides, designed to increase efficiency and reduce treatment periods, antibiotic-resistant infectious pathologies is one of the urgent problems of modern Bioorganic Chemistry and Pharmacy. The most interesting high antiherpes activity [1] has been found by scientists of the Institute of Bioorganic Chemistry of the Academy of Sciences of Uzbekistan among the synthesized azomethine derivatives of gossypol in terms of creating on their basis of drugs is gossypol compounds containing radical -CH2CH2SO3ONa (megosine).
Therefore, the aim of this work was to study how the initial hydrolysis azomethine derivative gossypol and study of the hydrolysis of the corresponding supramolecular complexes obtained by MACGA (monoammonium salt of glycyrrhizin acid) at a ratio of 1: 2 and 1: 4 (we called megaferon). Just study the admissibility of hydrophobic bases of local origin in the technology of ointment with a soluble form of megosine.
EXSPERIMENTAL PART
Synthesis of azomethine derivative gossypol was performed as follows:
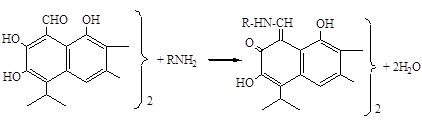
It is known that aqueous solutions of the Schiff base is unstable and hydrolyze to the original amines and aldehydes [2]. This property is also common for the azomethine derivatives of gossypol in 30 minutes in an aqueous solution. For the synthesized compounds in UV spectroscopy, there is an intense absorption maximum at 247.5 nanometre (nm) and a broad absorption band in the range 320-440 nm with the main peak at 402 nm.
Considering hydrolysis azomethine gossypol derivatives in aqueous solutions, the supramolecular complex was obtained synthesized substance monoammonium glycyrrhizin acid salt (MACGA) in various ratios (Picture 1, Picture 2).
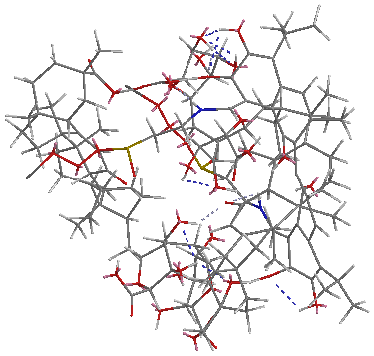
Picture 1. Supramolecular complex megaferon MACGA with a ratio of 1: 2

Picture 2 Supramolecular complexes megosine MACGA with a ratio of 1: 4
Interesting is the fact that the quantum-chemical calculations (calculation was performed using the MM2) in the case of a supramolecular complex megosine with MACGA in the ratio 1: 2, if the molecule megosine is above the molecule MACGA then, if the ratio of 1: 4 molecule megosine is in MACGA cavity.
The main reason for creating drugs based glycyrrhizin acid (GA) and its derivatives, it is well expressed solubilizing property. Many poorly soluble or water-insoluble substance drugs (aspirin, indomethacin, etc.) Are well dissolved in water in the presence of even a small amount of glycyrrhizin acid. The cause of the solubilizing properties of this natural substance is, of course, the intermolecular interaction that occurs when contact glycyrrhizin acid with various organic substances in the solution [3].
A comparative examination of the UV spectra of aqueous solutions and megosine megaferon (supramolecular complex with megosine MACGA) shows that as the storage solution is observed first a decrease and then increase with time absorbance intensity while maintaining the overall picture of the spectrum.
To investigate the degree of hydrolysis filmed UV spectra obtained supramolecular complexes in the SF-26 spectrophotometer at 10 min., 24 hrs. And 48 hrs. In buffer solutions with pH = 2.62, 7.31 and 10.96. (Picture 3,4,5)
All spectra exhibit an intense absorption maximum at 242-252 nm. As picture storage solutions varies spectra: megaferone in case there is an increase in optical density at 275 nm (from 0.14 to 0.41), but the overall shape of the spectrum is not changed.
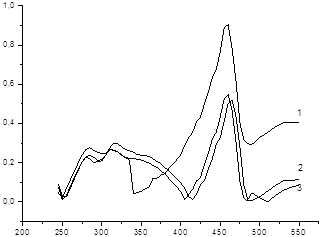
Pic.3.Megaferone pH = 2.62: 1 after 10 min 2 after 24-hour, 3-hours at 48..
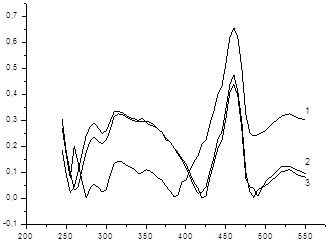
Pic.4 Megaferone pH = 7.31: 1 after 10 min 2 after 24-hour, 3-hours at 48..

Pic.5 Megaferone pH = 10.96: 1 after 10 min 2 after 24-hour, 3-hours at 48..
Thus, aqueous solutions of supramolecular complexes azomethine gossypol derivatives (in particular megosin) for 48 hours partially hydrolyzed. This property of supramolecular complexes is a push to create drugs. Preparation of supramolecular complexes of drugs and these drugs gives water-soluble property, and it increases the bioavailability of drugs. It is also known that the solubility of the drug can vary from the presence of adjuvants and used formulation technology. In the handbook of Mashkovskiy M.D. registered 3% ointment of megosine. On the basis of similar pharmacological action but Substance megaferone, we have developed the composition and technology of 3% ointment. [3] As an excipient used transesterificated cottonseed oil and tallow in a ratio of 48: 52 with the addition of up to 3% emulsifier T-2. The properties of this formulation excipients previously reported [4].
Prepared according to the rules of pharmaceutical technology, ointment megaferone on quality indicators has been studied in comparison with a standard sample, prepared on the basis of the Pharmacopeia. Results of the study are shown in Table 1.
Table 1
Comparable quality of megaferone ointment
|
View foundations |
Appearance |
The average pH value of |
Structural and mechanical properties |
|
|
centrifugation |
temperature control |
|||
|
Hydrophobic trans esterification base with an emulsifier T-2 |
Ointment homogeneous creamy consistency of a light brown color and odor |
6,5 |
stable |
Ointment stable system stratification was observed |
|
Standard base vaseline - lanolin (9: 1) |
Ointment yellow uniform, creamy consistency |
6,3 |
stable |
Delamination was not observed |
The analysis results show that the proposed composition meets specification. Hence the use of a hydrophobic base of local raw materials is possible in soft medicinal forms technology. Next, we have developed a method of quantitative determination of active substances in the ointment megaferone respectively against megosine and glycyrrhizin acid. Since the wavelength of the test substances in a very close distance, we found it necessary to develop a new method by combining the methods of TLC and UV spectrophotometry.
Separation and megosine GA in a thin layer of sorbent were performed under the above conditions. Method of chromatospektrofotometrical development was as follows: at the starting line of the chromatographic plates "Silufol - UV254" using the calibration applied to the capillary test solution A in a volume of 1 ml. In parallel to the chromatography were subjected to standard samples of material witness megosine and GA. The plate was chromatographed in a solvent system of acetonitrile: methanol: water (5: 5: 5) ascending manner. Once a mobile phase had reached the front, the plate was removed from the chamber and dried at room temperature. adsorption zones detected in the UV - light at λ = 254 nm. For quantification, localization zone eluted transferred onto a paper filter and washed with appropriate solvents. Zone megosine localization and washed with 25 ml of GA 100 ml of purified water.
Preparation A test solution megaferone: 1,5g ointment of megaferone in a glass beaker was added 50 mL of purified water was heated in a water bath until dissolved. The resulting solution was allowed to cool, then filter paper was separated and the aqueous portion was placed in a volumetric 100 mL flask was added 25 ml of ethanol. The solution is then adjusted with purified water to the mark.
Preparation of the standard solution megosine: 0.025 g megosine standard volumetric flask was placed in 100 ml of ethanol was added in a volume of 25 ml. Megosine after complete dissolution, the solution was brought to the mark with purified water (solution A).
Preparation of the standard solution of GA: 0.12 g GA standard was placed in a volumetric flask was added 100 ml ethanol in a volume of 25 ml. After complete dissolution of GA solution is brought up to the mark with purified water (solution A).
The optical densities of the eluates was measured with a spectrophotometer «Agilent 8453E» at a wavelength of 253 nm to 249 nm, and GA respectively. In parallel, the absorbance of standard solutions megosine and GA. Using these metrics using a formula to quantify the content of the test components. The results are shown in Table 2.
Table 2.
Quantitative determination megaferone using chromatospektrofotometrical method and metrological characteristics
|
№№ |
Hinge Megaferone, gr |
X gr |
Metrological characteristics |
|
GA |
|||
|
1 |
0,1511 |
0,1249 |
Хav=0,1256; f=4; t(P;f)=2,78; S2=0,0000016 ; S=0,0012649 ; ΔXav=0,00157 ; εav=1,25 % |
|
2 |
0,1497 |
0,1239 |
|
|
3 |
0,1503 |
0,1254 |
|
|
4 |
0,1528 |
0,1272 |
|
|
5 |
0,1537 |
0,1266 |
|
|
Megosine |
|||
|
1 |
0,1511 |
0,0262 |
Хav=0,02592; f=4; t(P;f)=2,78; S2=0,0000005 ; S=0,0007071 ; ΔXav=0,001965 ; εav=3,3 % |
|
2 |
0,1497 |
0,0258 |
|
|
3 |
0,1503 |
0,0249 |
|
|
4 |
0,1528 |
0,0256 |
|
|
5 |
0,1537 |
0,0271 |
|
The results in table show that the average relative error of the chosen method was to megaferone 2.6%, which is typical for substances supramoleculic polyphenol compounds. Comparison of the results showed the acceptability of the use of these methods in chemical analysis of soft medicinal forms of polyphenols.
CONCLUSIONS:
As the initial hydrolysis 1.Izuchen azomethine gossypol derivative and corresponding supramolecular complex obtained based MASGK (mono ammonium salt of glycyrrhizin acid) in a ratio of 1: 2 and 1: 4 (called contact megaferon). Thus detected, aqueous solutions supramolecular complex compounds for 48 hours are subjected to partial hydrolysis. This property of supramolecular complexes is a push to create drugs. Preparation of supramolecular complexes of drugs and these drugs gives water-soluble property, and it increases the bioavailability of drugs.
2. It is also studied the possibility of preparation of ointments using hydrophobic bases of local origin. Preliminary tests have shown compliance of the proposed composition of the regulatory requirements on the soft dosage forms. Biopharmaceutical study conducted by Kruvchinsky, the results of which will be provided in the following publications.
2. G.A. Tolstikov, Yu.I.Murinov, L.A.Baltina, Chemical Pharm. Magazine, 24 (9), 26-27 (1990).
3. M.D.Mashkovsky, Medicines, Vol.1, Publishing House of the medical literature (1998) .- S. 362.
4. Ya.K.Nazirova, H.M.Kamilov, K.S.Mahmudzhanova, Chemical Pharm. Magazine., 43 (10), 99-101 (2009)
Haitbaev A.H., Nazirova Ya.K., Abdurakhmanov M.M. STUDIES TO INTRODUCE MEGOSINE SUPRAMOLECULAR COMPLEXES WITH GLYCYRRHIZIN ACID TO MEDICAL PRACTICE. International Journal Of Applied And Fundamental Research. – 2016. – № 5 –
URL: www.science-sd.com/467-25088 (25.02.2026).











 PDF
PDF