About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Biological sciences
Abstract. Rapidly expanding multidrug-resistant microorganisms stimulate an intensive study of natural and man-made antimicrobial peptides activity. In recent years, antimicrobial effect of natural, evolutionarily balanced mixtures of biologically active substances, containing antimicrobial peptides is more and more investigated. A special place among these complexes take salivary cells secret of medicinal leech, primarily providing high efficiency hirudotherapy, which has been actively used since ancient times, and in recent periods. We first investigated the antimicrobial activity of this secretion in vitro against mycobacteria Mycobacterium smegmatis. It is shown, that the action of the natural complex on the cells is enhanced over time, resulting in about a day to a significant loss of viability of a population of cells and lysis. The picture of mycobacterial cell morphology disturbances was studied using different methods of electron microscopy during their lysis influenced by Hirudo medicinalis cells salivary secretion. It was concluded, that we need to study this natural complex activity on different, including - and MDR, tuberculosis strains of pathogens with the aim of science-based definition of prospects for secretion of salivary of medicinal leeches cells at certain stages of complex treatment of tuberculosis and other diseases.
Keywords: mycobacterium; antimicrobial peptides (AMP); the secret of the salivary cells of the medicinal leech Hirudo medicinalis (SSK MP); Scanning and transmission electron microscopy (SEM and TEM).
Introduction. Microorganisms' multi-drug resistance is rapidly expanding in recent years, and recognized as an urgent problem of global significance. The UN has pledged to control the fight with this problem [9]. Of particular concern are drug resistant pathogens infections such as tuberculosis. Mycobacterium tuberculosis Mycobacterium tuberculosis infected about one third of the world's population, and approximately every second there is a new case of infection. According to WHO, 9 million people are getting TB per a year worldwide, of which 3 million die from its complications. In Russia, the dispensary is more than 300 thousand people of tuberculosis patients, in many cases - MDR, which pathogens are resistant to the most effective anti-TB antibiotics [3, 13].
Tuberculosis mycobacterium differs in survival within macrophages. Infection process of M. tuberculosis is characterized by "unfinished phagocytosis" - the growth of mycobacteria inside macrophages as a result of violation of function of lysosomes, the impossibility of phagolysosome formation ending with macrophages loss. Then mycobacterium spread to other cells of the infected person. It is precisely the control of the macrophages fate that is critical in determining the outcome of infection with tuberculosis battle between pathogens and the human immune system [13]. Despite intensive study for more than 130 years of tuberculosis pathogens and ways of dealing with them, only recently synthesized M. tuberculosis toxin was discovered and explored, leading to macrophage death. The latter refers to a new class of toxins capable synthesized not only by mycobacterium tuberculosis, but also by more than 600 species of bacteria and fungi, including such pathogens as Listeria, Yersinia pestis. [14] This opens up prospects for the development of more effective ways to treat not only tuberculosis and other infections, but also cardiovascular disease, as many studies have convincingly demonstrated the causal link with TB incidence and development of most of them [3, 8, 12]. However, recent studies initiated that toxin kills macrophages has not led to the creation of new strategies for the treatment of tuberculosis. While for its treatment they offer, for example, the use of the "right" combination of known anti-TB drugs, selected from five of their groups. However, these methods do not always lead to the desired results. [4]
As an alternative traditionally used antibiotics, which are rapidly developing resistance of mycobacteria and many other pathogens, is considered natural and artificially produced antimicrobial peptides (AMP). Often an obstacle to the therapeutic use of AMP is their high hemolytic activity, as well as toxicity. In addition, many out of AMP - is the most important link of innate immunity of microorganisms, so called owners. Therefore, in the process of co-evolution of bacterial pathogens and their hosts a number of microbes developed the ability to counteract some of the AMP. Recently proved, that only the combination of several antimicrobial proteins and / or peptides does not cause the emergence of resistance in pathogens [17]. In connection with this study of antimicrobial activity of natural, evolutionarily balanced complexes containing AMP is expanding. A special place among these complexes take the secret of the salivary cells of the medicinal leech Hirudo medicinalis (SSK MP) - the primary humoral agent, providing high efficiency of treatment with leeches - hirudotherapy. Hirudotherapy has multifactorial anticoagulant, thrombolytic, anti-ischemic, anti-microbial, immune-stimulating, restores microcirculation effect, and a number of other effects, due to which has been it is used successfully in the present time in the complex treatment of cardiovascular and other diseases, as well as in microsurgery, after reconstructive and other operations for the effective recovery of microcirculation in venous stasis [2, 16]. SSK MP is not toxic and does not cause hemolysis. It includes more than a hundred well-known and not yet studied proteins and AMP, as well as around 200 small molecules. One of the most important and well-studied components of the SSK is AMP destabilase-lysozyme. The SSK is usually contained several isoforms of this enzyme, other types of lysozymes, other lytic enzymes [2]. Destabilase-lysozyme, as other lysozymes, exhibits high antimicrobial activity to a range of fungi and bacteria [19, 20]. However, mycobacteria is normally resistant to lysozyme [6, 11].
Previously, we first briefly reported on the sensitivity of Mycobacterium smegmatis to the antibiotic effect of SSK MP solution and the stability of the bacteria to the effects of recombinant destabilase-lysozyme from the medicinal leech [7]. As is known, M. smegmatis is generally used as a model system for the study of mycobacteria since this type virtually pathogenic, although it has more than 2,000 homologous genes with M. tuberculosis and specific wall structure of mycobacteria cell [5].
The aim of this study was to investigate the antimicrobial activity of SSK MP in relation to the M. smegmatis by conventional bacteriological methods, as well as the study by electron microscopy pictures of cell morphology violation of mycobacteria during their lysis influenced SSK MP.
Material and methods. Were selected native accuring, free of impurities, from the SSK MP H. medicinalis by previously described method [10]. The protein concentration of SSK in physiological solution is determined according to the method of Bradford. The work was carried out with the culture of M. smegmatis of 377pieces in the Museum of Cultures Department of Microbiology, Moscow State University of M.V. Lomonosov (MDMSU). Bacteria were grown at 30 ° C during the day on an agar nourishing environment with the following composition: K2HPO4 - 7,0; KH2PO4 - 2,0; Sodium citrate - 0.4; MgSO4 - 0,05; (NH4) 2SO4 - 1,0; H2O Dist .; pH 7,0 - 7,2. K medium supplemented with 10 g / l Tryptose Broth and 10 g / l glucose; and 15 g / l of highly purified agar ( «Sigma, USA). In this same environment was determined the antimicrobial activity of solutions of a known concentration of protein in the agar diffusion method, considering the size of the inhibition zone test culture growth from the end of the hole. The minimum inhibitory concentration (MIC) was determined by integrating solutions antibiotic dilution series and activities were expressed as pg / ml of protein solution. The viability of mycobacteria is determined in a known manner by counting the colonies grown from preserved cell viability after the impact on them of the studied solutions. Statistical processing of the results was performed using Microsoft Excel programs; significance of differences p <0,05.
Preparation of test microorganism M. smegmatis for transmission and scanning electron microscopy was performed by conventional methods as described in our previous work [10]. The sections of fixed cells in epon, as well as preparations made by negative contrast, were viewed in the TEM Geol 1011, (Japan) at an accelerating voltage of 80 kV. The shape and surface of the material was examined by SEM «JSM-6380" (Japan) and «CamScan» (UK) at an accelerating voltage of 20 kV.
Results and discussion.
Fig. 1 shows a SEM picture of a TEM morphology changes and M. smegmatis cells as a result of their effect on the growth in liquid medium SSK MP solution (50 ug / ml). After 3 hours, the influence of such cells to form aggregates, which are connected via mucosal cilia. TEM (Figure 1, photo 5 and fig. 1, photo 6) shows the distortion of the structure of cell membranes, cell wall separation, aggravated by incubation time and ending with the destruction of the cell membranes.
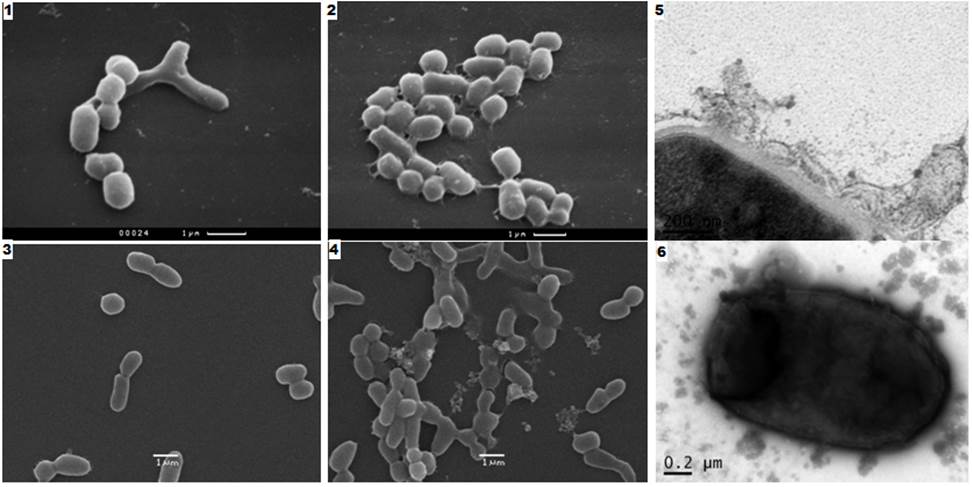
Fig. 1. Type of Mycobacterium smegmatis cells after their MP SSK antibiotic effect on the growth (50 ug / ml, 30 ° C). SAM: 1), 3) - control, the smooth surface of cells (two different experiments); 2) - with impaired cell adhesion surface (after 3 hours of SSK MP); 4) - lysis of the adherent cells, the cytoplasm out (after 11 hours of action SSC CHM); TEM (after 11 hours of action SSK MT): 5) peeling, destruction of cell membranes; 6) vesicles on the surface and within the cell; cytoplasm output ie its components (contrast agent).
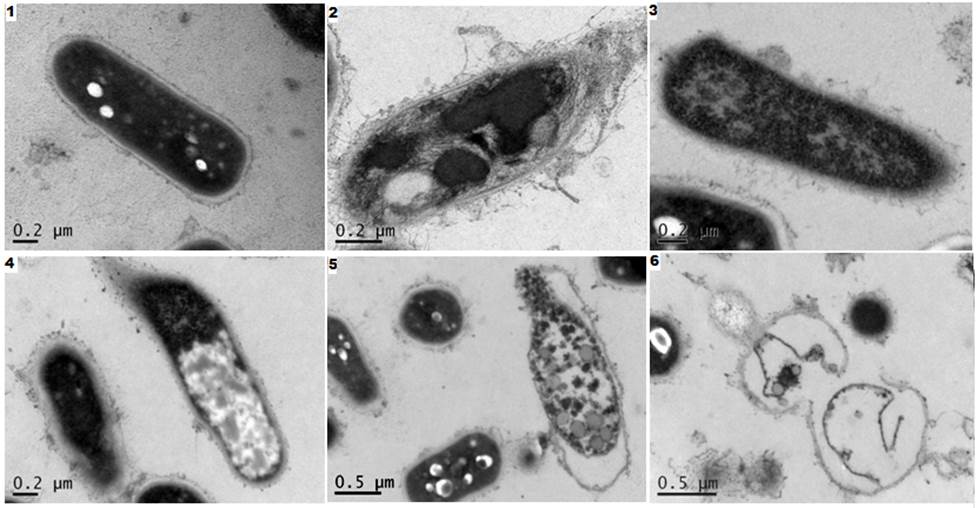
Fig. 2. Slice types of Mycobacterium smegmatis cells in TEM after antibiotic effect on their growth MP SSK (50 ug / ml, 11 h 30 ° C). SEM: 1) - control, cells with a smooth surface, dark cytoplasm; 2) separation of the cell wall, the permeability of a violation of the cytoplasmic membrane, resulting in a broken internal composition of the cell; 3) -5) enlightenment and cytoplasm output in place of the destroyed cell membranes; 6) the remains of membranes to lyse the cells.
Visible vesicles on the surface of cells, intracellular contents output due to violations of selective permeability of the cytoplasmic membrane and its subsequent lysis.
Fig. 2 shows the pattern in the TEM sections are lysed under the influence of SSK MP M. smegmatis cells after 11 hours of growth. It can be seen that the cells are at different stages of lysis. This phenomenon is characteristic of bacterial populations consisting of dissociates, whose sensitivity to antimicrobial agents depends on the thickness of the cell walls and other factors, and therefore varies several times, it was analyzed in detail and proved previously [18].
We tested the viability of mycobacteria cells after the effect of SSK MP solution on them (Table 1).
Table 1. Survival of M. smegmatis cells after SSK MP solution (50 ug / ml)
|
Solution duration SSK MP, h |
% of lost cell viability |
|
3 |
9,5 ± 0,8 р< 0,05 |
|
11 |
63,2 ± 7,1 р< 0,05 |
After 3 hours of such activity, only about a tenth of the mycobacterial cells lost viability. After 11 hours, these cells were already more than half the population.
The minimum inhibitory concentration SSK MP solution used by us in respect of M. smegmatis was 17 ug / ml for a day before incubation.
Thus, the tested species of mycobacteria are sensitive to the antibiotic effect of SSK MP which increases for about 1 day.
On the suppression of a number of infectious pathogens in hirudotherapy previously reported, indicating, in generalized form, the importance of the latter as a possible additional source of treating a large number of diseases, including - infectious, cardiovascular, as well as in microsurgery [1]. Extract patented use of many types of body parts and leech salivary cells of Hirudinidae family as an antimicrobial agent, but at the same mycobacterium was not shown as an example of sensitive microorganisms [15].
Specific results of the loss of viability of mycobacteria, the description of the picture cell lysis M. smegmatis under SSK MP solutions first obtained in our research.
Results.
The sensitivity of our findings to the M. smegmatis antibiotic effect of SSK MP solution indicate the need for a deeper study of the action of this solution not only to the M. smegmatis, but also on the different strains of tuberculosis pathogens of humans and animals. This is important for the purpose of determining the use of scientifically sound prospects MP SSK at some stages of complex treatment of tuberculosis and other diseases - especially in patients with weakened immunity, impaired functioning of the cardiovascular and other systems.
2. Baskova I.P. Hirudotheraphy scientific basements. Humoral link. 2015. Tula. «Akvarius». 228 p. (In Russian).
3. Broxmeyer L. Heart disease: the greatest 'risk' factor of them all. Med. hypotheses. 2004. 62(5):773-779.
4. Caminero J.A., Sotgiu G., Zumla A., Migliori G.B. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010. 10(9):621-629.
5. Chacon O., Bermudez L.E., Zinniel D.K., Chahal H.K., Fenton R.J., Feng Z., Hanford K., L. Adams L.G. and Barletta R.G. Impairment of D-alanine biosynthesis in Mycobacterium smegmatis determines decreased intracellular survival in human macrophages Microbiology. 2009. 155. 1440–1450.
6. Cimino M., Alamo L. and Salazar L. Permeabilization of the mycobacterial envelope for protein cytolocalization studies by immunofluorescence microscopy. BMC Microbiology. 2006. 6:35 – 39.
7. Guo Danyan, Pavlova I.B. Comparison of the antibiotic action of the salivary secretions of the medicinal leech cells and enzyme destabilase-lysozyme, which is part of the secret. Medical academic journal, St. Petersburg. (In Russian). 2012. 12. 5. p. 485-486.
8. Huaman M.A., Henson D., Ticona E. , Sterling T.R. and. Garvy B.A. Tuberculosis and cardiovascular disease: linking the epidemics. Tropical Diseases, Travel Medicine and Vaccines. 2015. 1 :10. DOI: 10.1186/s40794-015-0014-5.
9. Mushtaq A. UN commits to tackling antimicrobial resistance. Lancet Infect Dis. 2016. 16(11):1229-1230.
10. Pavlova I.B., Yudina T.G., Baskova I.P., Guo Danyan, Chen L.С., Feoktistova H.A.,
Vasilyev D.A., Zolotukhin C.H. Studying of prospects of use of the secret of salivary cages of the medical bloodsucker of Hirudo medicinalis and preparation «Piyavit» as the antimicrobic complexes which aren t causing resistance in microorganisms. Modern problems of science and education. (In Russian). 2015. №2. URL: www.science-education.ru/131-23637 (Accessed 3th December 2015).
11. Sethi D., Mahajan S., Singh C., Lama A., Hade M.D., Gupta P. and . Dikshit K.L Lipoprotein LprI of Mycobacterium tuberculosis Acts as a Lysozyme Inhibitor. Journal of Biological Chemistry. 2016. 291. 2938-2953.
12. Sheu J.-J., Chiou H.-Y., Kang J.-H., Chen Y.-H. and Lin H.-C. Tuberculosis and the Risk of Ischemic Stroke. A 3-Year Follow-Up Study. Stroke. 2010. 41: 244-249.
13. Silva C., Perdigão J., Alverca E., Jordao L. Exploring the Contribution of Mycobacteria Characteristics in Their Interaction with Human Macrophages. Microscopy and Microanalysis 2013. 19:1159 – 1169.
14. Sun J., Siroy A., Lokareddy R.K., Speer A., Doornbos K.S., Cingolani G., Niederweis M. The tuberculosis necrotizing toxin kills macrophages by hydrolyzing NAD. Nature Structural & Molecular Biology. 2015. 22. 672–678.
15. Tasiemski A, Salzet M. US Patent No. 20,120,251,625. Washington, DC: US Patent and Trademark Office, issued October 4; 2012. Use of extract of leeches as antibacterial agent. WO 2011/045427 A1, 2011.
16. Whitaker I., Maltz M., Siddall M.E., Graf J. Characterization of the Digestive Tract Microbiota of Hirudo orientalis (Medicinal Leech) and Antibiotic Resistance Profile. Plastic and Reconstructive Surgery. 2014. 133(3):408e-18e.
17. Yu G., Baeder DY., Regoes RR., Rolff J. Combination Effects of Antimicrobial Peptides. Antimicrob. Agents Chemother. 2016. 4. 60(3): 1717 - 1724.
18. Yudina T.G., Bryukhanov A.L., Zalunin I.A., Revina L.P., Kostina L.I., Voyushina N.E., Chestukhina G.G., Netrusov A.I., 2007. Antimicrobial activity of the different proteins and their fragments from Bacillus thuringiensis parasporal crystals against clostridia and archaea. Anaerobe, 13(1). 6-13.
19. Yudina T.G., Danyang Guo, Piskunkova N.F., Pavlova I.B., Zavalova L.L., Baskova I.P. Antifungal and antibacterial functions of medicinal leech recombinant destabilase-lysozyme and its heated-up derivative. Frontiers of Chemical Science and Engineering. 2012. 6(2), 203-209.
20. Zavalova L.L., Yudina T.G., Artamonova I.I., Baskova I.P. Antibacterial Non-Glycosidase Activity of Invertebrate Destabilase-Lysozyme and of Its Helical Amphipathic Peptides. Chemotherapy, 2006. 52:158-160.
Pavlova I.B., Guo Danyan, Chen L.C., Yudina T.G. SENSITIVITY OF MYCOBACTERIUM SMEGMATIS TO ANTIBIOTIC EFFECT OF SALIVA CELLS SECRETION OF MEDICAL LEECH HIRUDO MEDICINALIS. International Journal Of Applied And Fundamental Research. – 2016. – № 6 –
URL: www.science-sd.com/468-25200 (15.02.2026).











 PDF
PDF