About Us
Executive Editor:Publishing house "Academy of Natural History"
Editorial Board:
Asgarov S. (Azerbaijan), Alakbarov M. (Azerbaijan), Aliev Z. (Azerbaijan), Babayev N. (Uzbekistan), Chiladze G. (Georgia), Datskovsky I. (Israel), Garbuz I. (Moldova), Gleizer S. (Germany), Ershina A. (Kazakhstan), Kobzev D. (Switzerland), Kohl O. (Germany), Ktshanyan M. (Armenia), Lande D. (Ukraine), Ledvanov M. (Russia), Makats V. (Ukraine), Miletic L. (Serbia), Moskovkin V. (Ukraine), Murzagaliyeva A. (Kazakhstan), Novikov A. (Ukraine), Rahimov R. (Uzbekistan), Romanchuk A. (Ukraine), Shamshiev B. (Kyrgyzstan), Usheva M. (Bulgaria), Vasileva M. (Bulgar).
Chemical sciences
Abstract. The presented material describes the main characteristics of a chemical element, such as lead, the first mention of which some historians refer to III – II, others - to the VII millennium BC. The lead content in the earth's crust is about 0.0016%. One of the most important ores from which lead is mined is lead luster. Speaking about the distribution of lead in the biosphere, it should be noted that high concentrations of lead are found in many minerals and ores, in micro- and ultra-micronutrient quantities - in almost all objects of the surrounding world. The content of lead in the atmosphere depends on the place and time of sampling, as well as on weather conditions. Speaking about the effect of lead on biological objects, it should be noted that this component is a poison that affects all living things. Its compounds are dangerous not only by a pathogenic effect, but also by cumulative therapeutic effect, a high accumulation rate in the body, low speed and incomplete excretion with waste products. Lead in a state of molecular ion dispersion is especially dangerous. It is able to penetrate from the lungs into the circulatory system and from there is transported throughout the body. In connection with the foregoing, it should be noted that lead is a serious environmental pollutant, having an adverse effect on humans, animals, plants, and on the self-purification of water bodies and the work of sewage treatment plants. Everything speaks about the relevance of the subject matter.
A method of obtaining new modified sorbents based on flasks of Astrakhan region is presented. A comparative study of the sorption of lead ions on the surfaces of the sorbents was carried out. Also, the static adsorption isotherm of substances from aqueous solutions was studied. The change of enthalpy (ΔH), isobaric-isothermal potential (ΔG) and entropy (ΔS) of sorption and kinetics of lead ions sorption from water solutions were calculated. The results of the study can be used for the water purification from lead ions.
Keywords: sorbent, sorption, lead, heavy toxic elements, flocculants, water purification.
1. Introduction
Lead is an element of the main subgroup of group IV (6th period) of the periodic system of elements created by D.I. Mendeleev. The first mention of it is referred by some historians to III – II, by others to the 7th millennium BC. In Pliny's texts lead is called plumbumnigrum. So, because of its name up to the 17th century it wasn’t sometimes distinguished from tin, known in the Middle Ages under the name of plumbumalbum.
The lead content in the Earth’s crust is 0,0016% (by mass). One of the most important ores from which lead is mined is lead glance 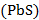 . Speaking about the lead distribution in the biosphere, it should be noted that the crust contains
. Speaking about the lead distribution in the biosphere, it should be noted that the crust contains 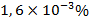 of this component by mass. According to the data from various authors sources, the cosmic abundance of this element varies from 0,47 to 2,9 atoms per
of this component by mass. According to the data from various authors sources, the cosmic abundance of this element varies from 0,47 to 2,9 atoms per 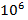 silicon atoms. The corresponding value for the Solar System is 1,3 atoms per
silicon atoms. The corresponding value for the Solar System is 1,3 atoms per 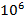 silicon atoms.
silicon atoms.
Lead is found in high concentrations in many minerals and ores, but almost all objects of the surrounding world contain this element in micro and ultramicro quantities. The lead content in the atmosphere depends on the place and time of sampling, as well as on weather conditions. The average concentration of lead in the air of industrial cities is 2,5-4,5 µg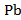 /
/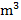 and the same mark in the air of villages is 0,5 µg
and the same mark in the air of villages is 0,5 µg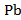 /
/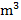 .
.
Other objects, such as rainwater, open source water, sea water, open ocean surface, water, soil, sedimentary clay minerals, plants contain certain amounts of the component (% by mass): 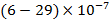 ,
, 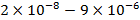 ,
, 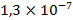 , –
, –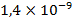 ,
, 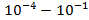 ,
, 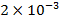 ,
, 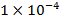 respectively.
respectively.
At the same time the amount of lead (in tons) in the atmosphere is 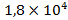 , in soils –
, in soils –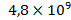 , in sedimentary deposits –
, in sedimentary deposits –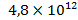 , in the oceans waters –
, in the oceans waters –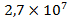 , in the waters of rivers and lakes –
, in the waters of rivers and lakes –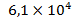 , in subsoil waters –
, in subsoil waters –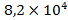 , in the water and land organisms: living
, in the water and land organisms: living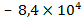 , dead –
, dead –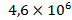 .
.
The MPC of lead for drinking water is 0,1 mg/dm3; for the air – 0,3 mg/dm3; for drinking mineral waters – 0,3 mg/dm3.
Speaking about the effect of lead on biological objects, it should be noted that this component is a poison that affects all living organisms. Its compounds are dangerous not only by the causative effect, but also by the cumulativeness of the therapeutic effect, the high accumulation rate in the body, the low rate and incompleteness of excretion with waste products.
It was found that even at a concentration of 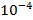 % in soil, lead inhibits the activity of enzymes, and highly soluble compounds are particularly harmful in this respect; the presence of lead in water at a concentration of
% in soil, lead inhibits the activity of enzymes, and highly soluble compounds are particularly harmful in this respect; the presence of lead in water at a concentration of 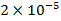 % is disastrous to fish, even low concentrations of lead in water reduce the amount of carotenoid and chlorophyll in algae.
% is disastrous to fish, even low concentrations of lead in water reduce the amount of carotenoid and chlorophyll in algae.
In addition, 200 thousand cases of lead poisoning among children as a result of ingesting paint pieces based on lead compounds have been registered in the United States. Also, according to the results of 10-year statistics, a correlation between the number of deaths from lung cancer and the elevated levels of lead and other metals in the air of industrial enterprises areas in the Federal Republic of Germany that consume coal has been established.
The degree of toxicity of the component depends on the concentration, physicochemical state and nature of lead compounds. Lead in a state of molecular ion dispersion is especially dangerous. Lead can penetrate from the lungs into the circulatory system and then it can be transported throughout the body. Although the component and its inorganic compounds act similarly, toxicity is growing more symbatically because of their solubility in biological fluids of the body. This does not diminish the danger of poorly soluble compounds that change their state in the intestine which leads to subsequent increase in their absorbability.
According to the authors, lead ions that are bound to the electron-donating sulfur atoms of the sulfhydryl groups poison enzymes. It is the main reason which leads to the inhibiting of many enzymatic processes in the body by the component. When lead intoxication occurs, serious changes in the nervous system, thermoregulation, blood circulation and trophic processes take place, as well as the immunobiological properties of the organism and its genetic apparatus undergo changes.
In the initial phase of poisoning a disturbance of conditioned reflex activity is observed and then disorders of the sympathetic and parasympathetic nervous system occur together with the possible manifestations of insomnia, hallucinations and seizures. Lead concentrations that are less than the MPC are considered harmless and don’t cause clear signs of poisoning. The MPC for organic lead compounds in the air is 0,005 mg/m3. Therefore, diagnostic tools and therapies are still valuable.
High density, softness, easy workability, relatively low conductivity, corrosion resistance and the ability to react with organic substances make lead especially valuable for practical purposes. It is used for radiation protection, as a structural material in the chemical industry, for the manufacture of protective coatings for electrical cables and battery electrodes. Large amounts of lead are used to manufacture various alloys with metals such as bismuth (coolant in nuclear technology), tin, small additives of gold and copper (to produce solders for the manufacture of printed circuits), antimony, tin and other metals (to produce solders and alloys for printing and antifriction purposes).
Based on the foregoing, it should be noted that lead is a serious environmental pollutant that impact the most harmful effect on humans, animals, plants, as well as on the processes of self-purification of water objects and the work of treatment facilities. Thereby the considered topic and the researches of this field is undoubtedly relevant today [1-15].
2. Experimental
The study presents the methods of producing the modified sorbents based on Astrakhan area flasks. The possibility of water purification from lead ions by using the sorbents was studied. Designed sorbents can be used for drinking water in the network of domestic water supply and in individual water purifiers intended for drinking, cooking and the use of economic-technical purposes [16-19].
A method of producing a sorbent SV-1-A. 100 g of portland cement-500, 10 g of micronized manganese dioxide (MnO2), 25 cm3 of 10% solution of sodium chloride are added to 100 g of finely divided flask with approximately 0.01 mm in diameter particles (the deposit is village Kamenniy Yar of Astrakhan region), the resulting mixture is thoroughly stirred. The obtained mass is allowed to dry to the point at which the granules may be formed from it. The granules are dried at temperature of 100-1050С, then the product should be calcified for 3-4 days. The resulting material are allowed to stand in water as long as the reaction to a chloride ion will be negative and then it is dried at 100-1050С.
To create a sorbent with a large number of micropores in a mixture of «flask - Portland cement-500 – pyrolusite» a solution of sodium chloride was added. After washing the sodium chloride from the sorbent, the finished porous material is formed. It has a high sorption capacity and simultaneously high strength. The reason for introducing pyrolusite into the sorbent is to provide a sorbent which has oxidizing properties to low molecular weight organic and inorganic compounds, as well as to a large set of microorganisms [16-18].
A method of producing a sorbent-SV-1-A2. 100 cm3 of flocculent Z-92 is added to100 g of finely divided sorbent SV-1-A with approximately 0.01 mm in diameter particles. The resulting solution is thoroughly mixed and allowed to settle, the remaining liquid is decanted and then 500 cm3 of distilled water is filled to the mixture with constant stirring. The procedure is repeated once more, and then the sorbent is left for an hour. The resulting adsorbent is dried in a thin layer at temperature of 50-600С with constant stirring.
A method of producing a sorbent SV-1-A3. 100 cm3 of flocculent A-1510 is added to100 g of finely divided sorbent SV-1-A with approximately 0.01 mm in diameter particles. The resulting solution is thoroughly mixed and allowed to settle, the remaining liquid is decanted and then 500 cm3 of distilled water is filled to the mixture with constant stirring. The procedure is repeated once more, and then the sorbent is left for an hour. The resulting adsorbent is dried in a thin layer at temperature of 50-600С with constant stirring.
The study of the kinetics of sorption of lead ions on sorbents SV-1-A2 and SV-1- A3. 20 cm3 of lead solution of 1·10-3 М concentration was added to the 500 cm3 flask, and then the resulting solution was adjusted to 500 cm3 by distilled water. 20 g of finely divided sorbent was added to this solution while switching on a stopwatch, then the mixture was stirred quickly. The resulting solutions were studied at three different temperatures: 298, 277 and 313 K. In an appropriate time intervals, the muddy samples were taken which were filtered through a glass filter or were centrifuged. Sampling was carried out at regular intervals up to 30 minutes. Then 4 cm3 of an organic reagent PAR (4- (2-pyridylazo) resorcinol) was added into each solution in the test tubes. The obtained solutions were mixed, and their optical densities were measured at 530 nm in a cuvette of 0.5 cm in thickness relative to distilled water. The isotherms of sorption kinetics were constructed in the coordinates «optical density (A) – time (τ) » (Figure 1, 2).
3. Result and discussion
The study of the kinetics of sorption of lead ions included the construction of sorption kinetics isotherms by measuring optical densities of the solutions at different intervals of time, the calculation of the sorption rate constants, the change in entropy of the activated complex formation (DS)#, the activation energy of the formation of the adsorption complex (Еact). The isotherms of sorption kinetics in the coordinates «optical density (A) – time (τ)» are presented in Fig. 1, 2.
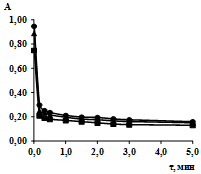
Fig. 1. Sorption kinetics isotherms of lead ions by sorbent SV-1 A2: -Δ- 277 K; -□- 298 K; -○- 313 K
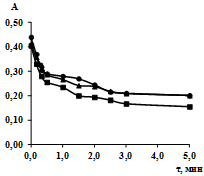
Fig. 2. Sorption kinetics isotherms of lead ions by sorbent SV-1 A3: -Δ- 277 K; -□- 298 K; -○- 313 K
The time for establishing the equilibrium of sorption is a necessary characteristic of describing the equilibrium processes, and the value of the entropy change of activation of the formation of the activated complex is necessary to establish the mechanisms of sorption of lead ions by the examined sorbents.
According to the research the sorption kinetics constants of lead ions on the modified sorbents at temperatures of 277, 298 and 313 K, S# и Еact , were calculated:
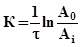 , (1)
, (1)
where А0 – the initial optical density; Аi – optical density of the solution at the moment of time τ; τ – time, s.
According to the Arrhenius curves in the coordinates «lnK – 1/T» the values of activation energy of sorption kinetics (Еact) was calculated and the entropy change of sorption complex formation (S#) was also calculated using the Eyring equation:
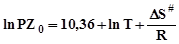 , (2)
, (2)
In equation (6) PZ0 – is a pre-exponential factor in the Arrhenius equation, ?S# – the entropy change of activation of activated complex formation, R – gas constant, T – temperature.
The results of calculations of the sorption kinetics constants, Еact и ?S#, of lead ions on the modified sorbents are shown in table 1.
Table 1
The thermodynamic characteristics of sorption kinetics of lead ions on sorbents SV-1-A2
and SV-1-A3 (n=6, Р=0,95, tp=2,57)
|
Defining characteristics |
Temperature, К |
Sorbents |
|
|
SV-1-А2 |
SV-1-А3 |
||
|
Rate constants К×10-2 s-1 at temperatures, К |
277 |
1,93±0,1 |
0,39±0,1 |
|
298 |
1,94±0,1 |
0,76±0,1 |
|
|
313 |
1,84±0,1 |
0,35±0,1 |
|
|
Еакт, кJ/mol |
In the range of 277 to 313 K |
5,81±0,5 |
3,80±0,3 |
|
-ΔS#, J/mol×К |
277 |
1,84±0,2 |
2,02±0,2 |
|
298 |
1,85±0,2 |
2,03±0,2 |
|
|
313 |
1,86±0,2 |
2,04±0,2 |
|
Almost all studied sorption processes can be characterized by fairly steep initial line segment of sorption kinetics isotherms. As can be seen from the results of experiments, sorption is rapid enough, and finishes for three minutes, that means that the sorbate is almost completely adsorbed on the examined sorbents.
4. Conclusions
The results obtained in the study clearly demonstrate the high efficiency of the use of modified sorbents created on the basis of Astrakhan region flasks for water purification from toxic heavy metals such as lead. The information above suggests the expediency of using the natural resources of Astrakhan region - flasks and sorbents based on it for solving some of the environmental problems associated with purification of natural and waste waters from toxic heavy metals such as lead.
2. Akhmetov N.S. General and inorganic chemistry / Textbook. for universities. – 4th ed., Corr. – P.H.: Higher. school, ed. center "Academy". – 2001. – 743 p. – illustrations.
3. Glinka N.L. General chemistry. – Ed. 15th, rev. – Publishing House "Chemistry". – 1971. – 712 p. – 145 fig. – 31 tab.
4. Spitsyn V.I., Martynenko L.I. Inorganic chemistry / Part II. Textbook. – Publishing House of Moscow State University. – 1994. – 624 p. – illustrations.
5. Lidin R.A., Molochko V.A., Andreeva V.V. Chemical properties of inorganic substances: Proc. manual for universities. 3rd ed., Corr. / Ed. Lidina R.A. – P.H.: Chemistry. – 2000. – 480 p. – illustrations.
6. Bolshakov K.A. Chemistry and technology of rare and trace elements. – Study manual for universities. – Ed. 2nd, reedit. and add. – P.H.: Higher School. – 1976. – 368 p. –illustrations.
7. Alekseev Yu.V. Heavy metals in soils and plants. – P.H.: Agropromizdat. – 1987. – 142 p.
8. Bandman A.L., Gudzovsky G.A., Dubeykovskaya L.S. and others. Harmful chemicals. Inorganic compounds of elements of I-IV groups. – P.H.: Chemistry. – 1988. – 512 p.
9. Grushko I.M. Harmful inorganic compounds in industrial wastewater. – P.H.: Chemistry. – 1979. – 160 p.
10. Basics of general industrial toxicology. / Ed. N.A. Tolokontseva, V.A. Filova. – P.H.: Medicine. – 1976. – 304 p.
11. Pertsovskaya A.F., Panikova E.L., Velikanov N.L. Effect of heavy metals on soil biosystems depending on its pH / hygiene and sanitation. – № 4. –1987. – P. 14-17.
12. Sokolov O.A., Chernikov V.A. Atlas of HM distribution in environmental objects. –P.H.: Pushino. –1999. –164 p.
13. Chemistry of industrial wastewater / Ed. A. Rubin. –P.H.: Chemistry. –1983. – 360 p.
14. Lazarev N.V., Gadaskina I.D. Harmful substances in industry. – P.H.: Chemistry. –1977. –V. 3. – 607 p.
15. Shachneva E.Yu. Effects of heavy toxic metals on the environment // «Scientific potential in the modernization of services in the region». – Interuniversity collection of scientific articles. – Astrakhan. – GAOU JSC VPO «AISI». - №2 (3). – 2012. – P. 127-134.
16. Shachneva E.Yu. Physical chemistry of the adsorption of flocculants and synthetic surfactants on sorbent SV-1-A: Abstract of Cand. Chem. Sciences: 02.00.04. – Makhachkala. – 2011. – 23 p.
17. Shachneva E.Yu. Physical chemistry of the adsorption of flocculants and synthetic surfactants on sorbent SV-1-A: Dis. cand. Chem. Sciences: 02.00.04. – Astrakhan. – 2011. – 139 p.
18. Shachneva E.Yu., Archibasova D.E. Adsorption of cadmium ions from aqueous solutions on modified sorbents Chemistry Chemical Technology. – Vol. 12. – No. 2. – 2018. – p. 182-187.
Shachneva E.Yu., Kudinova D.E., Khentov V.Ya. DETERMINATION OF KINETIC PARAMETERS OF THE PROCESS OF ADSORPTION OF LEAD IONS ON A NATURAL MINERAL SORBENT. International Journal Of Applied And Fundamental Research. – 2018. – № 5 –
URL: www.science-sd.com/477-25502 (03.03.2026).











 PDF
PDF